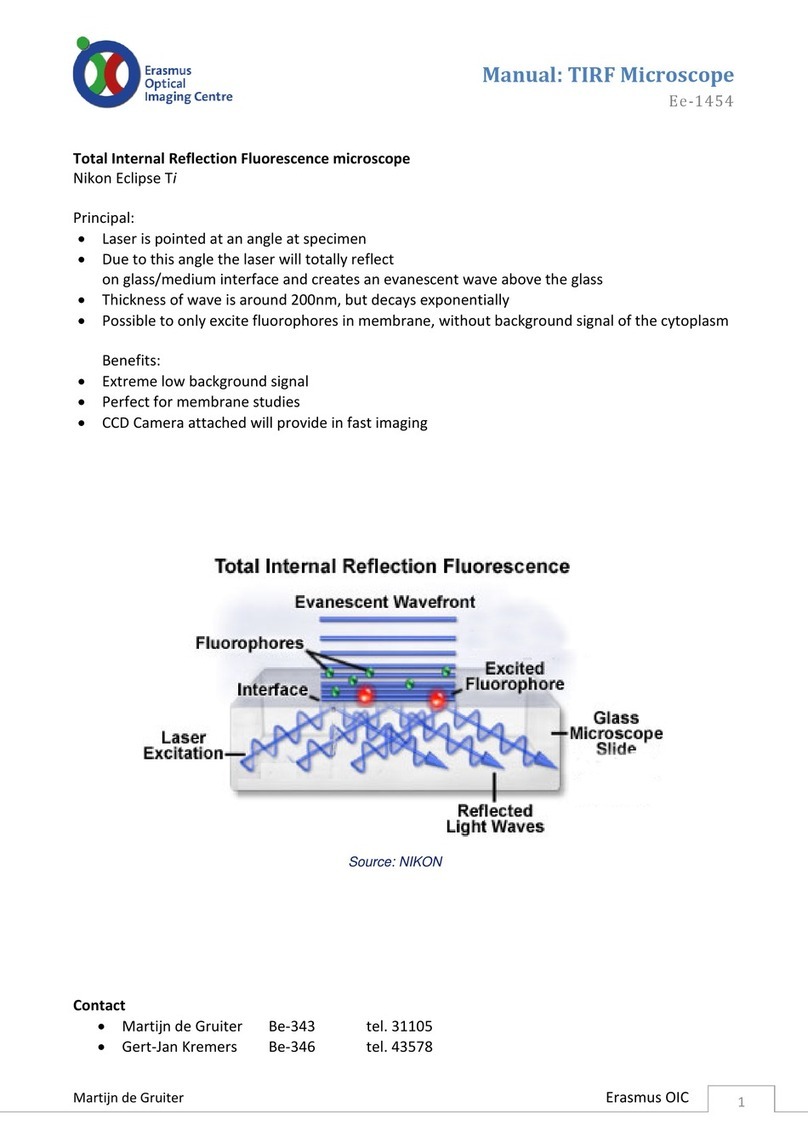
CARE AND MAINTENANCE
oCleaning the lenses
To clean the lens surfaces, remove dust
using a soft hair brush or gauze. Only for
removing finger marks or grease, should
soft cotton cloth, lens tissue or gauze
lightly moistened with absolute alcohol
(methanol or ethanol) be used.
For cleaning the objectives and immersion
oil use only xylene.
Observe sufficient caution in handling
alcohol and xylene.
f)Cleaning the painted surfaces
Avoid the use of any organic solvent (for
example, th inner, ether, alcohol, xylene
etc.) for cleaning the painted surfaces and
plastic parts of the instrument.
8Never attempt to dismantle!
Never attempt to dismantle the instrument
so as to avoid the possibility of impairing
the operational efficiency and accuracy.
OWhen not in use
When not in use, cover the instrument with
the accessory vinyl cover, and store it in a
place free from moisture and fungus.
It is especially recommended that the
objectives and eyepieces be kept in an
airtight container containing desiccant.
oPeriodical checking
To maintain the performance of the instru-
ment, we recommend to check the instru-
ments periodically. (For details of this
check, contact our agency.)
3
CONTENTS
I. NOMENCLATURE 4
II. ASSEMBLy 6
III. PREPARATION 8
1. Interpupillary Distance Adjustment 8
2. Diopter Adjustment 8
3. Optical Path Change-over in the
Trinocular Eyepiece Tube "TP" 8
4. Centering the Objectives 8
5. Centering the Condenser Lens 9
6. Orientation of the Dia-polarizer 9
IV. MICROSCOPY 10
1. Operating Procedure 10
2. Manipulation of Each Element 11
1) Focusing 11
2) Condenser aperture diaphragm 11
3) Field diaphragm 11
4) Circular graduated stage 11
5) Objectives 11
6) Eyepieces 12
7) Achromat strain-free condenser 12
8) Bertrand lens 12
9) 1/4 A&tint plate 13
10) Dia-polarizer and analyzer _ 13
11) Filters 14
12) Illumination system 14
V. PHOTOMiCROGRAPHy 15
VI. ACCESSORIES 17
1. 5enarmont Compensator 17
2. Quartz Wedge 17
3. Monocular Eyepiece Tube "AP" 18
4. Epi-illuminator "M" 18
5. Attachable Mechanical Stage Type "E". 19
VII. TROUBLE SHOOTING TABLE 20
REFERENCE 23
ELECTRIC SPECIFICATIONS 23