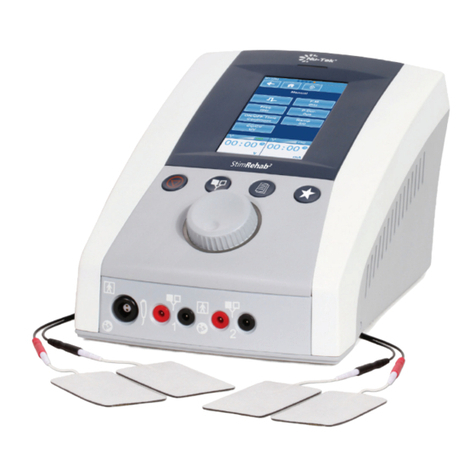
6
1. Always use this device under the directions of a physician.
2. Patients with the following diseases, symptoms or conditions should
not use the device:
● During pregnancy or menstrual cycle.
● Acute disease, heart disease, tubercle disease, facial neuralgia (sharp
facial pain), pernicious tumor, hemophilia, high fever, abnormal blood
CAUTIONS
9. The device should be used only with adapter recommended for use by the
manufacturer.
10. Do not modify this equipment without authorization of the manufacturer.
11. Do not service and maintain the device while it is in use.
12. The device must only be served ,repaired and opened by individuals at
authorized sales centers.
13. Do not use the device if it is damaged. The continuous use of a damaged
device may cause injury, improper results, or serious danger.
14. Do not store the device at an extremes temperature (below -10°C or over
50°C) or extremes humidity (below 20%RH or over 93%RH). Failing to do
so may aect the performance of the device.
15. Store the device in the dry, clean place. Keep the device away from pet
and pests.
16. Do not expose the product to any chemical solvent, water lint, dust, direct
sunshine or high temperature.
17. Keep unit out of the reach of young children. The wire can cause
strangulation.
18. Keep the device out of the reach of children to avoid inhalation or
swallowing of small parts.
19. Do not operate the device when connected to any other medical device.
20. Do not operate this device in an environment where other devices used
intentionally radiate electromagnetic energy in an unshielded manner.
21. Please check the cables regularly. If the cables are damaged, please stop
using it.