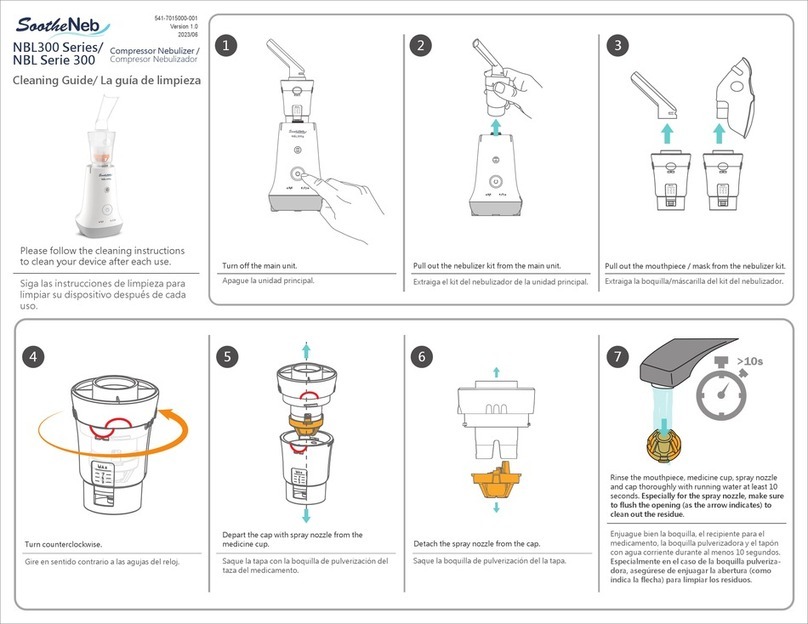
O-Two Equinox Relieve User manual

Made in Canada by O-Two Medical Technologies Inc.
Part Number: 15PL2175 -Rev. 14 - July 2023
USER
MANUAL
Equinox® Relieve
NITROUS OXIDE/OXYGEN ANALGESIC
GAS MIXING AND DELIVERY SYSTEM
01EQ1000/01EQ1000F


TABLE OF CONTENTS
1. Introduction
1.1. Indications for Use
1.2. Contraindications
1.3. Product Description/
Principles of Operation
1.4. Warranty Information
1.5. Safety Precautions
1.6. Performance Specification
2. Preparation for Use
2.1. Component List
2.2. Pre-Use Functional Checks
3. Operating Instructions
3.1. Connection of Hoses
3.2. On/O Selector
3.3. Demand Valve
3.4. Low Input Pressure Alarms
3.5. Low Input Pressure Shut O
3.6. Fail Safe Protection
3.7. Output Mixture Monitoring
4. Servicing
4.1. Routine Maintenance
4.2. Cleaning
5. Replacement Parts & Accessories
4
4
4
5
6
7
8
9
9
9
11
11
12
12
12
13
13
14
15
15
16
17

4Equinox® Relieve - User Manual
1. Introduction
1.1. Indications for Use
The O-Two Equinox® Relieve N2O/O2Analgesic Gas Mixing and
Delivery System is intended for delivering a 50/50% mixture
of nitrous oxide and oxygen, on demand, to a conscious,
spontaneously breathing patient.
The device is suitable for use in:
• Pre-hospital (ambulance) use, and
• In-hospital use (ER, Labor and Delivery etc.)
1.2. Contraindications
The contraindications for this device include, but may not be
limited to:
• Hypersensitivity to the medication
• Head injuries with impaired consciousness
• Maxillofacial injuries
• Artificial, traumatic or spontaneous pneumothorax
• Air embolism
• Middle ear occlusion, ear infection
• Decompression sickness
• Abdominal distension / intestinal obstruction
Note: Nitrous Oxide/Oxygen (N2O/O2) mixtures must never
be used in any condition where air is trapped in the body and
expansion (up to 3x original size) would be dangerous. For
example, it will exacerbate pneumothorax and increase pressure
from any intracranial air. Air in any other cavities such as the
sinuses, middle ear and gut may also expand.

5
Equinox® Relieve - User Manual
1.3. Product Description/Principles of Operation
The device provides connectors for connection with nitrous oxide
and oxygen cylinders through pressure regulators. The device has
only one control for turning ON or OFF the device. When it is
turned ON, the output of N2O/O2gas mixture will only be activated
by an inspiratory eort by the patient. The output of N2O/O2gas
mixture is pre-set at 50/50%. Neither the patient nor medical
personnel are able to adjust, eliminating the risk of delivering a
hypoxic mixture.
The gas specific built-in alarm systems will generate both visual
and audible alarms should either the nitrous oxide or oxygen
input drop to 40 PSI, and the device will be automatically shut o
should either the Nitrous Oxide or Oxygen input drop to 35 PSI.
Both visual indicator and audible alarm circuits for both gasses
are powered by oxygen only to prevent dumping nitrous oxide
gas into atmosphere during alarm cycling.
The device is also equipped with a primary “failsafe” circuit (3.6)
that will activate a continuous alarm and shut o the device should
the output pressure dierential from the mixer exceed 2 psi.
If either the Nitrous Oxide or Oxygen Supply runs out or is shut
o, the device will automatically shut o; however the patient will
be able to breathe atmospheric room air through the emergency
air intake.
Note: The O-Two Equinox® Relieve System is considered a critical
device, and its components considered critical components.
It shall only be used by patients under the guidance of those
individuals trained in the operation of nitrous oxide/oxygen
analgesic gas delivery systems. Thoroughly review the instruction
manual before use.
The 01EQ1000F is fitted with an oxygen enrichment button to
allow for the flushing of the system after use.

6Equinox® Relieve - User Manual
1.4. Warranty
WARRANTY
O-Two Medical Technologies Inc. products are manufactured from
the finest quality materials. Each individual part is subject to strict
quality control tests to ensure exceptionally high standards. The
manufacturer warrants to the purchaser of the O-Two Equinox®
Relieve N2O/O2Analgesic Gas Mixing and Delivery System
that its component parts are free from defects in material and
workmanship for a period of two years from the date of purchase.
The manufacturer will replace and /or repair all parts of the device
at its option for two years from the date of purchase at no cost to
the purchaser, upon the notification of the defects, in writing by
the purchaser. All shipping costs shall be borne by the purchaser.
The manufacturer shall be liable under this warranty only if the
device and its parts have been used and serviced in the normal
manner described in the instruction manual. There are no other
expressed or implied warranties. This warranty gives no specific
legal rights. You may also have other rights which may vary
according to local regulations.

7
Equinox® Relieve - User Manual
1.5. Safety Precautions
The O-Two Equinox® Relieve System is a self-administered device.
It shall only be used by patients under the guidance of qualified
personnel trained in its use. Carefully read this manual prior to
operation and use.
The following precautions should always be observed:
WARNING
1. When the unit is in use, do not smoke or use near open flame
either during operation or when changing the cylinder.
2. Never allow oil or grease to come into contact with any part of
the cylinder, regulator or Equinox® Relieve System. The device
has been degreased for oxygen service prior to delivery.
3. Equinox® Relieve System is designed for patient self-
administration.
4. Never attach the facemask to the patient using a head harness.
5. If the alarm sounds continuously, immediately discontinue use
and shut o the gas supply.
6. It is recommended that an oxygen monitor be used with this
device to monitor the gas output. However, if there is a concern
that the device has not undergone regular maintenance, at the
prescribed intervals, in accordance with this manual, then an
oxygen monitor shall be used.
7. Medical gases must be dry and free from dust and oil.
8. The Nitrous Oxide cylinder should be operated in an upright
position. If the Nitrous Oxide cylinder is in a valve-down
position while the post valve is open, liquid may be expelled
through the vent passages. This liquid Nitrous Oxide can cause
burns by freezing on exposed skin.
9. It is recommended to use cylinders that are at least 1/4 full.
Always turn on cylinder valve slowly and fully. When not in
use, always turn o the cylinder.
10. After use, always ensure that a gas cylinder with sucient
volume is attached before returning the unit to its normal
storage position.
11. After use, always ensure that all components are cleaned in
accordance with the instructions provided in this manual.
CAUTION:

8Equinox® Relieve - User Manual
1.6. Performance Specifications
12. Do not disassemble any part of the unit except where
described in this manual as any unauthorized disassembly will
invalidate the warranty.
13. Before use on a patient, the oxygen concentration of the
delivered gas should be checked.
14. US Federal Law restricts this device to sale by or on the order
of a physician.
Minimum Flow rate 120 L/min
Exhalation Resistance 0 to 6 cmH
2
O @ 60 L/min
Inhalation Resistance 0 to -6 cmH
2
O @ 60 L/min
Oxygen Concentration 42.5 to 57.5%
Oxygen Enrichment Flow
(01EQ1000F only) 30 L/min
Input Pressure 50 to 70 PSI (3.5 - 4.8 Bar)
Low Input Pressure Alarm 40 ± 1 PSI (2.7 Bar)
Device Shut-o Pressure 35 ± 4 PSI (2.7 Bar)
Failsafe protection
~ 2 PSI dierential pressure between O
2
and N
2
O
output gas. Prevents the output of high nitrous
oxide concentrations
Demand Valve Triggering
Pressure -1.5 to -2.5 cmH
2
O
Supply Hose Connections Refer to Section 6
Device Input Connections Nitrous Oxide: CGA1040 DISS
Oxygen: CGA1240 DISS
Patient Connector 15/22 mm
Patient Circuit (Single use)
Dual limb circuit with one-way valves and
19 mm Scavenging Port Connection on the
exhalation limb.
Patient Valve Dead Space 32 ml
Operating Temperature 41
0
F to 104
0
F (5
0
C to 40
0
C)
Storage Temperature -40
0
F to 140
0
F (-40
0
C to 60
0
C)
Weight 3.3 lb. (1.5 Kg)
Dimensions W x D x H 8.9”x6.6”x3.9” 226x168x99mm)

9
Equinox® Relieve - User Manual
2. Preparation for use
2.1. Component List
1. Operating Manual
2. O-Two Equinox® Relieve System
3. Single Use Patient Circuit with Mouth Piece
4. Oxygen Supply Hose
5. Nitrous Oxide Supply Hose
6. Test hose
2.2. Pre-use Functional Checks
Along with the contents of the shipping cartons you will require
the following items to enable you to undertake the pre-use
functional check:
• Nitrous oxide and oxygen pressure sources with 60 PSI output
capable to provide a minimum of 100 L/min at no less than 42
PSI (2.9 Bar).
• A breathing simulator with 30 L/min demand flow over a
minimum of 1 second. As a simple option a 1 Liter calibrated
gas syringe may be used.
Leak Test
Having connected the supply hoses to the regulated gas supplies
(refer to section 3.1 for CONNECTION OF HOSES), ensure that the
O-Two Equinox® Relieve ON/OFF selector is in the OFF position
and turn on the N2O and O2supplies. Using a mild soap solution,
spray the input connections to the device to check for leaks. If
any leak is present, tighten the connection and re-test. Once no
leakage is confirmed, turn the ON/OFF control to the ON position.
Note: If any components are missing from the shipping carton,
immediately call the supplier quoting the packing slip number,
your original purchase order number and the description of the
item which is missing.
Note: Factory calibration of this device uses a +/- 5% V/V tolerance.
These Pre-Use tests are designed for the user to confirm function and
accuracy of the device while taking into account dierences in test
equipment and test procedures that may aect the results obtained.
Any devices with results outside of the tolerance stated in the chart
should be returned to an authorized service center for checking.

10 Equinox® Relieve - User Manual
Reverse gas flow test
To test the reverse flow, connect one gas at a time to the
corresponding input connector, spray the other input connector,
no bubbles allowed from this connector.
Function test
The following features of the Equinox® Relieve System can be
individually tested or measured using a calibrated pressure gauge
and flowmeter during the Pre-use Functional Check:
1. Demand Valve Function and Oxygen Concentration
Demand Valve function and oxygen concentration of the delivered
gas can be determined by the following procedure:
Note: To fully test this function it is necessary to have a supply
regulator with an adjustable output pressure and a release valve
(Not supplied). Checking of the alarm may be undertaken by
simply slowly closing the cylinder valve.
A. Connect an oxygen monitor to the output connector of the
Relieve using the “3-Way T” Connector test assembly supplied.
B. Utilizing a 1 Liter calibrated syringe, connect the syringe to
the patient connection on the patient circuit connected to T-
connector. Turn on the Relieve unit and activate the demand valve
by cycling the syringe until the oxygen reading on the monitor
has stabilized. Demand valve function is confirmed by a change
in the reading on the monitor.
Setting Acceptable Range
50% 40% – 60%
2. Low Input Pressure Alarms
With the adjustable inlet pressure regulator set to a supply
pressure of 50 PSI, gradually reduce the supply pressure of the
regulator to around 40 PSI while slowly releasing the gas until
you hear the Low Input Pressure Alarm activated. For the nitrous
oxide alarm the pulsed tone frequency is set at a low frequency
of approximately 60 BPM. For the low oxygen alarm, the pulsed
tone frequency is set at high frequency of approximately 120 BPM.
When low pressure alarm is activated, the corresponding Gas
Supply Status Indicator will also cycle at the same rate of the alarm.

11
Equinox® Relieve - User Manual
3. Low Input Pressure Shut O
Continue to decrease the outlet pressure of the regulator to
around 35 PSI, the device should automatically shut o, and at
the same time, in the event the N2O pressure drops below 35 PSI,
an additional continuous alarm will be activated.
4. Oxygen Enrichment Button (01EQ1000F only)
The oxygen flow and concentration can be measured at the
patient connection port using an O2monitor and flow meter.
3. Operating instructions
3.1. Connection of Hoses
The O-Two Equinox® Relieve System is designed to operate on
medical nitrous oxide and medical oxygen from either individual
gas cylinders or piped-in medical gas systems.
The inlet fittings on the device are non-interchangeable, DISS
fittings, specifically for nitrous oxide and oxygen.
The device is designed to operate under 50 – 70 PSI input
pressures for both gas supplies.
The nitrous oxide supply hose provided shall be attached to the
N2O input connection on the left side of the device. The oxygen
supply hose provided shall be attached to the O2input connection
on the right side of the device. Tighten the supply hoses “Finger
tight” only – DO NOT USE A WRENCH (fig 1).
WARNING
Using a wrench or excessive force in tightening the supply hose
may damage the seal or the thread of the connection.

12 Equinox® Relieve - User Manual
3.2. ON/OFF selector
The ON/OFF SELECTOR controls the device in either its normal
operating mode or shut o.
3.3. Demand Valve
The device is equipped with a Demand System enabling
spontaneously breathing patients to demand 50/50% nitrous
oxide and oxygen.
An inspiratory eort by the patient will open the demand valve
and 50/50% nitrous oxide and oxygen will flow to the patient at a
rate in line with their inspiratory eort.
3.4. Low Input Pressure Alarms
Gas Supply Status Indicators
Located on the front panel, the O2and N2O visual indicators turn
green when oxygen and Nitrous Oxide are supplied to the unit.
Used in conjunction with the Low Input Pressure alarms, these
indicators provide additional reference for the operator as to the
gas supply status.
1. Attach the input
hoses to the device.
Fig. 1 - Connecting the Supply hoses and Patient Circuit
2. Attach a new
patient circuit
to the device.
The patient circuit is attached to the gas outlet on the front panel
of the control module by simply pushing the 22 mm taper over
the outlet

13
Equinox® Relieve - User Manual
Low Input Pressure Alarms
At any time, if the input pressure of either gas drops to 40 PSI, a
visual and audible Low Input Pressure alarm will be activated. The
alarm will cycle 45 to 75 times per minute for Nitrous Oxide, or 90
to 150 times for Oxygen and the gas supply indicators will flash
Green to black.
3.5. Low Input Pressure Shut O
The device will automatically shut o should the input pressure of
either gas drop to 35 PSI. This indicates that the drive gas is now
exhausted to the point where the device will no longer function
correctly (see also the Note in 3.6).
3.6. Failsafe Protection
The O-Two Equinox® Relieve System is equipped with a primary
“Failsafe” protection circuit.
Should the output pressure dierential of the internal mixer valve
exceed 2 psi the “Fail Safe” protection circuit will activate, a
continuous alarm will sound and the device will shut o.
Note: The shut o alarms will cease to function completely should
the O2input pressure drop below 20 PSI.
If either the Nitrous Oxide or Oxygen Supply runs out or is shut
o, the device will allow the patient to breathe on atmospheric
room air through the emergency air intake.
Note: If the oxygen enrichment control is held depressed for an
extended period the device may alarm. Releasing the button will
cancel the alarm.
Oxygen Enrichment Button (01EQ1000F only)
With the ON/OFF switch set to ON, a constant flow of oxygen
will be supplied to the patient when the enrichment button is
depressed.

14 Equinox® Relieve - User Manual
3.7. Output Concentration Monitoring
An oxygen monitor with alarm should be used to monitor the
output of the N2O/O2mixture. The monitor “Tee” piece is to
be placed on the outlet of the mixer prior to the circuit being
attached (fig 2).
Note: In Use, the drop in low input pressure may cause a dierence
in output pressure and trigger the Failsafe protection. In this case
the shut o alarm will activate instead of the low pressure alarm.
When the device is turned o the Failsafe protection circuit is
deactivated. Once initiated the alarm will continue its constant tone
until the device is turned o or the pressure dierential is resolved.
Note: Placing an oxygen monitor in the above location will remove
the additional monitoring line from becoming an issue for the
patient if the “Tee” piece is placed proximal to the facemask.
Additionally, the one-way valve system in the O-Two Equinox®
circuit will prevent expired air from reaching the monitor.
Fig. 2 - Connecting the Oxygen Monitor
Equinox®
Relieve
Oxygen
Monitor

15
Equinox® Relieve - User Manual
4. Servicing
4.1. Routine Maintenance
To ensure proper operation of the O-Two Equinox® Relieve, regular
inspection and checking of the device and accessories for correct
function should be undertaken by a responsible member of sta.
It is recommended that the routine preventive maintenance
be carried out and the Equinox® Relieve mixer be returned to
O-Two Medical Technologies or its authorized service center for
maintenance and service every 2 years as follows:
Description Procedure Criteria Schedule By
PM Visual
inspection
User Manual
4.1 Monthly
checking
Device in work
order, gas tanks are
full, no missing item Monthly User
PM Leak test User Manual
2.2 No leak
observed Every 6
months User
PM Function
check User Manual
2.2 [1] - [4] Within
specification Every 6
months User
Servicing Level II
service Service
manual Meet product
specifications Every 2
years Manufacturer/
service center
Servicing Full service Service
manual Meet product
specifications Every 6
years Manufacturer
Monthly inspection
The device should be visual inspected and leak tested monthly to
ensure that the components and accessories are present, the gas
cylinders are full and the device is in working order.
Function check
The device should be checked for proper function and mixture
output concentration at least every six months, and more
frequently in high use applications. Malfunction unit should be
returned to the manufacturer or an authorized service center
since this product is not designed for field disassembly or service.
Unauthorized repairs will nullify the product warranty.
Level II service
The device shall be returned to the manufacturer or an authorized
service center for Level II service every 2 years.
Manufacturer full service
The device shall be returned to O-Two Medical Technologies for
Manufacturer Full Service every 6 years.

16 Equinox® Relieve - User Manual
4.2. Cleaning
Routine cleaning of the device shall be undertaken to maintain the
device in a clean condition.
The patient circuit and mouth piece of the device are intended
for single use and shall be discarded after each patient use in
accordance with local protocols and replaced with a new circuit.
All other components should be wiped clean with a mild soap
solution or hard surface disinfectant suitable for the materials of
manufacture of the device. Under no circumstances should the
complete unit be allowed to be soaked or immersed in cleaning
solutions.
Detailed cleaning procedure is as follows:
1. Ensure that the device is disconnected from the gas supply
source.
2. Remove N2O and O2input hoses and wipe clean with a mild
soap or hard surface disinfectant. Ensure no cleaning solution
enters the hoses.
3. Remove the single patient use circuit from the device and
dispose of safely in accordance with local protocols
4. The enclosure of the device can be wiped over with a soft
cloth and mild soap solution or hard surface disinfectant.
Ensure no cleaning solution enters the input fittings. If there
is ingrained contamination a soft bristled brush may be used.
5. Dry all components thoroughly.
6. Attach a new patient circuit and connect the unit to gas supply
to check function prior to packaging for emergency use.
WARNING
Do not attempt to clean and sterilize any components that are
designated as disposable.

17
Equinox® Relieve - User Manual
ORDER N0PART
01CV8028-Cs O-Two Single-Use Analgesic Gas Patient Circuit with Scavenger
Hose and Adapter with Mouthpiece (Case/10)
17MP1997 90o(Male to Female) adjustable Oxygen DISS elbow
17MP1998 90o(Male to Female) adjustable Nitrous Oxide DISS elbow
01FM4999 Universal Facemask (Case/12)
ORDER N0PART
01FV4303-AFNR O-Two 6 Foot (1.85 Meter) O2Supply Hose with AFNOR
probe and 9/16” DISS Nut Device Connection
01FV4303-AGA O-Two 6 Foot (1.85 Meter) O2Supply Hose with AGA
probe and 9/16” DISS Nut Device Connection
01FV4303-CZCH O-Two 6 Foot (1.85 Meter) O2Supply Hose with CZECH
probe and 9/16” DISS Nut Device Connection
01FV4303-DIN O-Two 6 Foot (1.85 Meter) O2Supply Hose with DIN probe
and 9/16” DISS Nut Device Connection
01FV4303-DISS O-Two 6 Foot (1.85 Meter) O2Supply Hose with 9/16 DISS
Nut and 9/16” DISS Nut Device Connection
01FV4303-UNFR O-Two 6 Foot (1.85 Meter) O2Supply Hose with UNIFOR
probe and 9/16” DISS Nut Device Connection
01FV4303-BM O-Two 6 Foot (1.85 Meter) O2Supply Hose with BRITISH
probe and 9/16” DISS Nut Ventilator Connection
5. Replacement Parts & Accessories
Oxygen Hoses*

18 Equinox® Relieve - User Manual
ORDER N0PART
01FV4303-AFN-N2O
O-Two 6 Foot (1.85 Meter) N2O Supply Hose
with AFNOR Gas Supply Fitting and N2O DISS
Nut Device Connection
01FV4303-AGA-N2O
O-Two 6 Foot (1.85 Meter) N2O Supply Hose
with AGA Gas Supply Fitting and DISS
Nut Device Connection
01FV4303-CZCH-N2O
O-Two 6 Foot (1.85 Meter) N2O Supply Hose
with Czech Gas Supply Fitting and DISS Nut
Ventilator Connection
01FV4303-DIN-N2O O-Two 6 Foot (1.85 Meter) N2O Supply Hose
with DIN Nut and DISS Nut Device Connection
01FV4303-DISS-N2O
O-Two 6 Foot (1.85 Meter) N2O Supply Hose
with DISS Gas Supply Fitting and DISS
Nut Device Connection
01FV4303-UNF-N2O
O-Two 6 Foot (1.85 Meter) N2O Supply Hose
with UNIFOR Gas Supply Fitting and
DISS Nut Device Connection
01FV4303-BM-N2O
O-Two 6 Foot (1.85 Meter) N2O Supply Hose
with BRITISH Gas Supply Fitting and
DISS Nut Device Connection
Nitrous Oxide hoses*
*Select the hose connection type for the country of use


Your Representative is:
O-TWO MEDICAL TECHNOLOGIES INC.
For your nearest Authorized O-Two Distributor
In North America call Toll Free 1-800-387-3405
SERIAL N
0
:
www.otwo.com
45A Armthorpe Road, Brampton, ON, Canada, L6T 5M4
Telephone: +1 905 792-OTWO (6896) N.A. Toll Free: +1 800 387 3405
Facsimile: +1 905 799 1339 Email: resuscitation@otwo.com
MedNet EC-REP IIb GmbH
Borkstrasse, 10
48163 Münster, Germany
EC REP
This manual suits for next models
2
Table of contents
Other O-Two Respiratory Product manuals