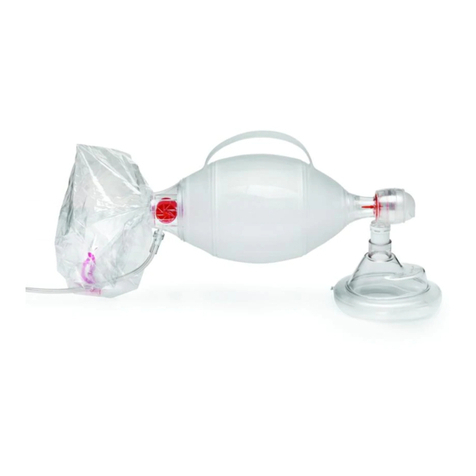
Pulmodyne VT Select User manual

Manual Resuscitator
INDICATIONS FOR MANUAL RESUSCITATOR USE:
• Pulmonary Resuscitation
CAUTIONS:
• This product must be used by qualied personnel in the techniques of
pulmonary resuscitation.
WARNINGS:
• Never store this resuscitator in a compressed state other than as delivered
by the manufacturer
• Verify pressure with a manometer at Manometer port.
• Do not occlude exit port of reservoir bag
• Resuscitators with PEEP valves should be used only qualied personnel.
• Device is packaged with the valve activated
• Always monitor the patient.
• Operating the resuscitator incorrectly can be hazardous
• Use the correct size resuscitator for the ideal body mass of the patient to
avoid the risk of hypoventilation or barotrauma.
• Avoid using an oxygen concentration more than that which is clinically
required by the patient. Delivering excessive oxygen can increase the risk
of oxygen toxicity e.g. pulmonary damage, retinopathy of prematurity
• Patient expired gas is potentially infectious. Breathing lters can reduce
but not eliminate contamination risk.
PRECAUTIONS:
DO:
1. Clear patient’s airway before using manual resuscitator.
2. Always check for proper function of resuscitator:
• Verify proper valve action.
• Verify that the valve is free of obstruction
• Verify patient is being ventilated by observing alternate rise and fall of
the patient’s chest and color of lips and face during resuscitation.
DO NOT:
1. Do not use in contaminated atomosphere (e.g., poisonous gases, smoke,
etc.)
2. If oxygen is used, do not use in presence of sparking equipment or open
ame.
3. Do not autoclave, gas, or chemically sterilize the Manual Resuscitator.
4. Do not lubricate ttings, connections, tubing, or other accessories of the
resuscitator to avoid the risk of re and burns.
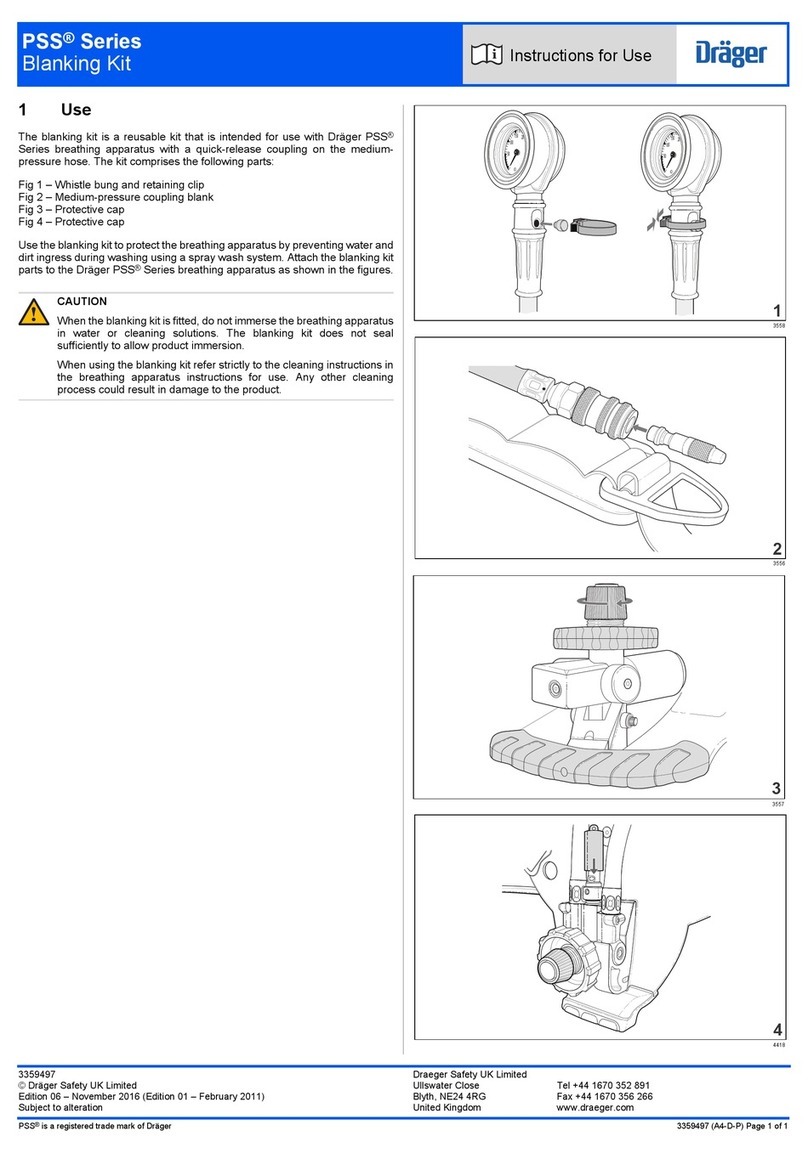
DIRECTIONS FOR USE
Set-up of Manual Resuscitator
1. Prior to using the resuscitator, visually verify proper valve action
while squeezing the resuscitator.
2. If resuscitating with high oxygen concentrations of oxygen, attach
oxygen tube to proper oxygen source.
3. Set oxygen ow on the order of a physician. Do not exceed 15lpm.
4. To attach resuscitation bag to mask, press mask’s 22mm I.D. into
resuscutator’s 22 O.D. connection.
5. When resuscitating through endotracheal tube or tube adapters,
remove mask and attach 15mm O.D. tube adapter to resuscitator.
This is a 15mm I.D. connection.
Operation of Manual Resuscitator-STANDARD SELECTION
1. Clear patient’s airway, if obstructed.
2. Tilt patient’s head back and pull chin up.
3. After establishing this position, place mask firmly over nose and
mouth and hold in place.
4. Resuscitate patient by alternatively squeezing and releasing the
bag at the prescribed rate.
• If faster cycling at reduced FiO is desired in the selected stan
dard setting unscrew tail end from the resuscitator to resuscitate
without oxygen
5. Verify that the patient’s chest rises and falls during resuscitation. If
movement is absent during resuscitation, check patient’s airway.
6. Time manual resuscitation with any spontaneous breathing to pre-
vent blockage of exhalation.
7. Clear valve obstructions, if any. Foreign material in the valve may be
removed by squeezing the bag briskly and shaking any remaining
obstruction of the exhalation port and/or rinsing with water.
8. Discard manual resuscitator after use
Operation of Manual Resuscitator-ACTIVATED SELECTION
1. Clear patient’s airway, if obstructed.
2. Tilt patient’s head back and pull chin up.
3. After establishing this position, place mask firmly over nose and
mouth and hold in place.
4. Resuscitate patient by alternatively squeezing and releasing the
bag.
5. When the activated valve is set, use one hand and place fingertips
on the ridges of the bag. Optional strap is included to help hold the
bag in place. when both the index finger and thumb come together.
Wait until the bag fully inflates before beginning the next breath.
6. Verify that the patient’s chest rises and falls during resuscitation. If
movement is absent during resuscitation, check patient’s airway.
7. Time manual resuscitation with any spontaneous breathing to pre-
vent blockage of exhalation.
8. Clear valve obstructions, if any. Foreign material in the valve may be
removed by squeezing the bag briskly and shaking any remaining
obstruction of the exhalation port and/or rinsing with water.
9. Discard manual resuscitator after use.
MPulmodyne, Inc.
2055 Executive Dr
Indianapolis, IN 46241 U.S.A.
www.pulmodyne.com
53036A-4-25-2022
1
Made in Malaysia

Resuscitator Specications:
Body Mass Range >40kg (Adult)
Operating Environmental Limits -18° C to 50°C
Storage Environmental Limits -40° C to 60°C
Exhalation Port 30mm
Patient Connection 15mm I.D. x 22mm O.D.
Maximum Bag Volume 1200mL
Delivered Volume Range (1 hand) 530mL (2 hand) 690mL
Deadspace (without mask) 9.6mL
Backward and Forward Leakage Negligible
Expiratory Resistance < 5 cm HO (0.5kPa) @ 50LPM
Inspiratory Resistance < 5 cm HO (0.5kPa) @ 50LPM
Resuscitator External Dimensions 301mm length/116mm diameter
Resuscitator Mass (w/o mask) 373 g
Delivered Oxygen Concentration (%) Average Values:
Oxygen Flow Rate
• Frequency (12 BPM)
• Tidal Volume (600mL)
5 10 15
Oxygen Concentration:
Standard 71 91 94
Activated 60 63 64
INTENDED USE FOR ADJUSTABLE PEEP VALVE:
• The postive end expiratory pressure (PEEP) valve is designed to be used in
conjunction with a manual resuscitation bag exhalation port to allow the user to
select positive end expiratory pressure from 5 to 20cm H2O. Attachment of this
valve requires no modication to either the resuscitator or valve. If PEEP is not
desired, remove PEEP valve.
CAUTIONS:
• This product must only be used by qualied personnel in the techniques of
pulmonary resuscitation.
• During use, the patient’s condition must be monitored.
• This product is not intended to be disassembled by the user; disassembly may
result in improper valve function.
WARNINGS:
• PEEP may produce adverse eects. Close observation and reliable assessments
are essential when using PEEP in patients with low circulatory blood volume,
impaired cardiac function, bullous lung disease or higher than normal lung
compliance. Assess the patient’s hemodynamic, ventilatory, and oxygenation
status whenever using PEEP.
• Use of sterilization or cleaning solutions may leave this product nonfunctional.
• This product is a one-way valve intended for respiratory use only and must not
be used on anesthesia circuits.
• Do not use PEEP valve if it becomes occluded. An occluded PEEP valve obstruct
patient’s exhalation and result in potential injury. Remove valve from exhalation
port and discard.
• Do not use PEEP unless you are qualied in the indications, benets, side eects,
contraindications, and goals of PEEP therapy.
• Do not use PEEP unless pressure is veried with a manometer.
DIRECTIONS FOR USE:
1. Prior to use, check to be sure the device is free of obstructions and verify proper
valve function.
2. Firmly seat the valve onto the resuscitation bag exhalation port.
3. The valve is furnished with graduations at approximate PEEP levels of 5, 10, 15,
and 20cm H2O. Using a manometer, verify PEEP settings prior to use.
4. Adjust the cap on the PEEP valve to acheive the desired PEEP levels. Clockwise
rotation will increase PEEP levels.
5. Observe the position below cap of the indicator markings in relation to the
pressure scale label to determine the approximate amount of PEEP being gener-
ated. Actual PEEP may vary with patient lung compliance and resistance. Verify
pressure with a manometer.
6. If PEEP valve becomes occluded, remove from exhalation port and discard. A
new PEEP valve can be readily attached to exhalation port.
7. Discard valve after use.
INTENDED USE FOR DISPOSABLE PRESSURE MANOMETER:
To provide visual indication of a patient’s airway pressure during ventilation. It may be attached to
the manometer port of proximal port on ventilation devices such as resuscitation bags, hyperina-
tion bags, CPAP masks, or CPAP circuits.
INSTRUCTIONS:
1. Attach the manometer with its exible connector onto the device’s manometer port.
2. Occlude the patient port on the attached device and pressurize the manometer to maximum
scale reading on the manometer.
3. Release the pressure and check that the oat returns smoothly back to “0”cm H2O mark.
4. Attach the system to the patient and monitor the patient to assure proper ventilation.
5. For a point of reference, set the “O”ring to be approximately at the desired pressure reading.
6. When repeated use is requiredfor the same patient, check the function and accuracy of the
manmeter prior to each use.
7. Discard after use.
WARNINGS:
• For single patient use. Do not clean or sterilize as this may aect the accuracy or function of
the manometer.
• Check function and accuracy prior to each use, including when repeated use is required for
the same patient.
• A minimal amount of leakage of ariway gas is normal. The eects of this leakage must be
evaluated for each patient.
CAUTIONS:
• This device must be used only by qualied personnel in the techniques of pulmonary resus-
citationor airway management.
• During use, the patient’s condition must be monitored. Should the performance of this de-
vice appear to be erratic during use, the device must be removed and replaced as required.
MANOMETER ACCURACY:
±1 cm H2O from 0-10 cm H2O
±2 cm H2O from 10-40 cm H2O
±3 cm H2O from above 40 cm H2O
OPTIONAL ACCESSORIES:
• PEEP VALVE
• DISPOSABLE PRESSURE MANOMETER
• FILTER
INTENDED USE FOR FILTER AEROPRO COMPACT STRAIGHT:
This Depth Filter is designed for use with ventilators, anesthesia machines and open ow systems
where ltration of inspired and/or expired gases is desired.
Product Specications and Information are listed in the table below.
Set Up:
1. Place the Filter in the circuit in the desired location, at the machine end, connected to the
Inspiratory outlet and/or expiratory inlet, or between the articial airway and the proximal
breathing circuit.
2. Attach gas sampling line to luer port, if present.
3. Ensure connections are secure.
4. Check for airow and function as part of circuit checkout procedure prior to use.
Cautions:
1. Do not resterilize, soak, rinse or reuse.
2. Ensure all connections are secure at all times.
3. Replace unit immediately if there is any contamination, occlusion or any indication of
malfunction.
4. The dead space of this product should be taken into consideration when determining tidal
volume and patient ventilation requirements.
5. Dispose of properly.
6. Federal (USA) law restricts this device to sale by or on the order of a physician.
Warnings:
• Replace Filter at least every 24 hours or earlier if increased resistance is noted.
Contraindications:
1. Filter should not be used in the proximal airway with patients producing fulminating frothy
secretions within their airways, or patients with hemoptysis.
2. Do not use in conjunction with conventional humidiers.
3. Do not add moisture to the Filter.
4. During delivery of inhaled medication the Filter should be removed or bypassed.
Product Resistance to Flow
(cmH2O @ L/min)
Dead
Space (mL)
Weight
(g)
Bacterial
Eciency
Viral
Eciency
Tidal Volume
(mL)
Aero-Pro Compact
Straight 2.0 @ 60 31.5 23.7 99.999 99.99 150-1000
SELECT VALVE POSITIONS:
STANDARD ACTIVATED
STANDARD: The valve has no restriction and is acting as
an unrestricted BVM.
ACTIVATED: The valve is restricted to produce a con-
trolled tidal volume and timed rell.
2
This manual suits for next models
5
Other Pulmodyne Respiratory Product manuals

Pulmodyne
Pulmodyne BiTrac MaxShield Select User manual

Pulmodyne
Pulmodyne BiTrac MaxShield Select 313-9524 User manual
Pulmodyne
Pulmodyne BiTrac HC User manual

Pulmodyne
Pulmodyne BiTrac Select User manual

Pulmodyne
Pulmodyne BiTrac Select 313-9001W User manual

Pulmodyne
Pulmodyne GO-PAP 313-4600 User manual