Ohio Medical, 1111 Lakeside Drive, Gurnee, Il 60031 (866) 549-6446 | Fax: (847) 855-6218
www.ohiomedical.com
—– 550542 (Rev.6) 06/2016
Page | 4
II. INSTRUCTIONS FOR USE
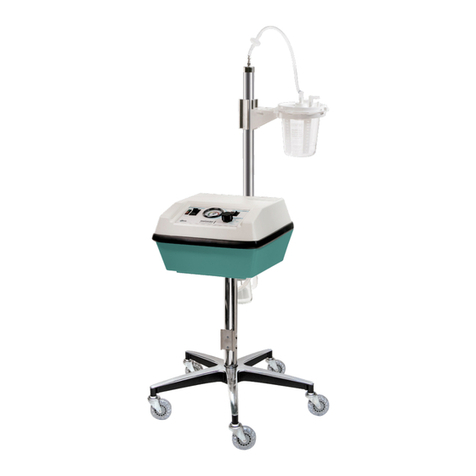
Please perform the following initial tests to ensure that your care-e-vac®3 is in good working
order and that no damage has occurred duringshipment.
A)
PreliminaryCheck
1. Visually inspect unit for physical damage that may have occurred during shipping.
2. Start without A/C power, depress the “VAC” switch to turn the unit ON and listen to
verify the pump starts and that the Green LED is on. If the red LED illuminates, re-
charge the battery per instructions in section II.C, Recharging Battery.
3. Occlude the vacuum port with your fingertip and adjust the regulator knob. Verify that
the gauge reflects a change in vacuum level while turning the regulator knob. Also,
verify the presence of vacuum at your fingertip. Adjust the regulator to full vacuum
ensure the gauge indicates “FULLVAC”.
4. Connect the AC power cord to the proper electrical outlet. Verify that the unit is
charging by ensuring the Amber LED ison.
5. The bottle bracket is adjustable to accept any collection device up to 1200 cc. The
bottle bracket can be adjusted by placing the unit on its side and locating the plastic
clamp, which secures the bracket. Using a Phillips head screwdriver, loosen (DO
NOT REMOVE) the screws and adjust the bracket to the desired position. Retighten
the screws.
The care-e-vac®3 is designed to use any size collection device up to 1200 cc. However,
if you are not using an Aeros disposable collection canister, make sure the collection de-
vice being used is equipped with a SAFETY OVERFLOW MECHANISM to protect the
pump from accidentaloverflow.
Ensure that you have the appropriate collection device, suction tubing and aspirator tip
(catheter) for patient use. The care-e-vac®3 is now ready to beused.
B)
Operating Instructions
1. Check the battery level by turning the unit on and verifying that the “OK” Green LED
illuminates. Turn the unit off.
2. Ensure that the hydrophobic filter is in-line between the vacuum port of the care-e-
vac®3 and the vacuum port on the collection bottle. If using another canister that
comes with a built-in filter, do not use external inline filter. Also, confirm the aspirating
tip is connected to the patient port on the collectionbottle.
3. Turn the care-e-vac®3 on and occlude to set, pinch the patient tubing closed, to adjust
the vacuum to the desired vacuum level by using the vacuum regulator knob. Set the
vacuum level to the least amount of vacuum necessary to properly suction the patient.