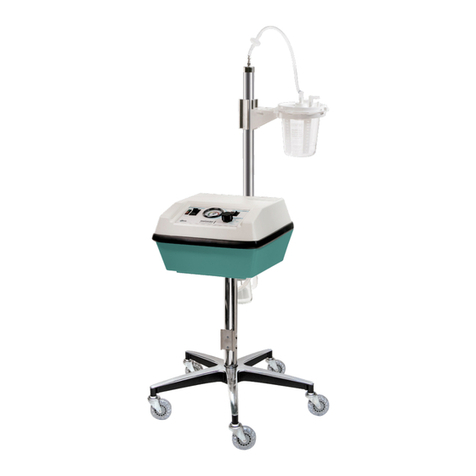
8700-0001-000 (Rev. 14) 11/18 5
1/Precautions
1.2 WARNINGS
Factory settings may be impacted during transport
therefore, the unit's timing cycle should be checked
prior to initial use and adjusted if necessary (see
Section 7.6 Timing Cycle Adjustment).
This device should be repaired only by qualied
Ohio Medical or Ohio Medical-trained, qualied
personnel, using only Ohio Medical recommended
parts. There are risks associated with using
anything other than Ohio Medical parts. Ohio
Medical will assume no responsibility for incidents
which may occur if the product was not repaired
in accordance with procedures authorized by Ohio
Medical.
If the vacuum regulator is repaired or disassembled
in any manner, the service checkout procedure
must be performed before using the equipment on
a patient.
After patient use, if regulator is contaminated then
handle in accordance with you hospital’s infection
control policy.
To reduce transportation personnel and/or service
personnel exposure to hazardous contamination,
DO NOT ship any suction equipment that has been
contaminated.
Do not use this device in the presence of ammable
anesthetics. Static charges may not dissipate and
a possible explosion hazard exists in the presence
of these agents.
Connection to positive pressure sources such as
oxygen and medical air, even momentarily, could
injure the patient or operator.
Ohio Medical will assume no responsibility for
incidents which may occur if the product is not
used in accordance with product labeling.
To help prevent aspirate from entering the device,
wall outlet and pipeline equipment, a safety trap
should be attached prior to its use. Aspirate in the
regulator, wall outlet and pipeline equipment may
impair its operations. The use of the safety trap
and suction lter will help prevent this and extend
the life of suction equipment.
1.3 CAUTION
Do not use any Loctite® products or any products
which contain Methacrylate Ester as an active
ingredient to seal the threads on the adapters/
probes and ttings.
Use of lubricants other than recommended may
degrade plastic or rubber components.
Do not steam autoclave or liquid sterilize the
regulator. Severe impairment to the operation
of the regulator will result. The only acceptable
method of sterilization is with gas (ethylene oxide).
Sterilization with ethylene oxide mixtures may
cause crazing (minute supercial cracking) of some
plastic parts. Crazing will be more pronounced
when mixtures containing Freon® are used.
Following sterilization with ethylene oxide, parts
should be quarantined in a well ventilated area to
allow dissipation of residual ethylene oxide gas
absorbed by the material. Aerate parts for 8 hours
at 54°C (130°F). Follow your hospital sterilization
procedure.
Do not use harsh chemical or cleaning solution. Do
not spray cleaners directly onto suction regulators.
Only use chemical recommended in this manual.
If any evidence of damage is found, repair as
necessary or contact your authorized service
provider.
Connection to positive pressure sources such as
oxygen and medical air, even momentarily, could
damage the equipment.
The suction control knob must be completely pushed
in to adjust the vacuum level. Failure to do so may
damage the vacuum regulator.
Not for transport use: The categories of eld
and transport user are specically dened in
ISO 10079-3. “Field” means use at accidents
or emergencies outside a hospital. “Transport”
means use in ambulances, cars and airplanes.
These situations may expose the equipment to
uneven support, water, dirt, and mechanical shock
and temperature extremes. Ohio Medical Suction
equipment has not been tested to comply with the
specic requirements of these categories.
Note: Ohio Medical requests that parties acquiring
this device:
Report the device’s purchase, receipt in trade,
return after sale, loss, destruction, or retirement.
Contact your Ohio Medical customer service
representative to obtain manual updates.
Authorized Service Center / Customer Service
Call 1-866-549-6446 or +1 847-855-0500 for service
and repair information.