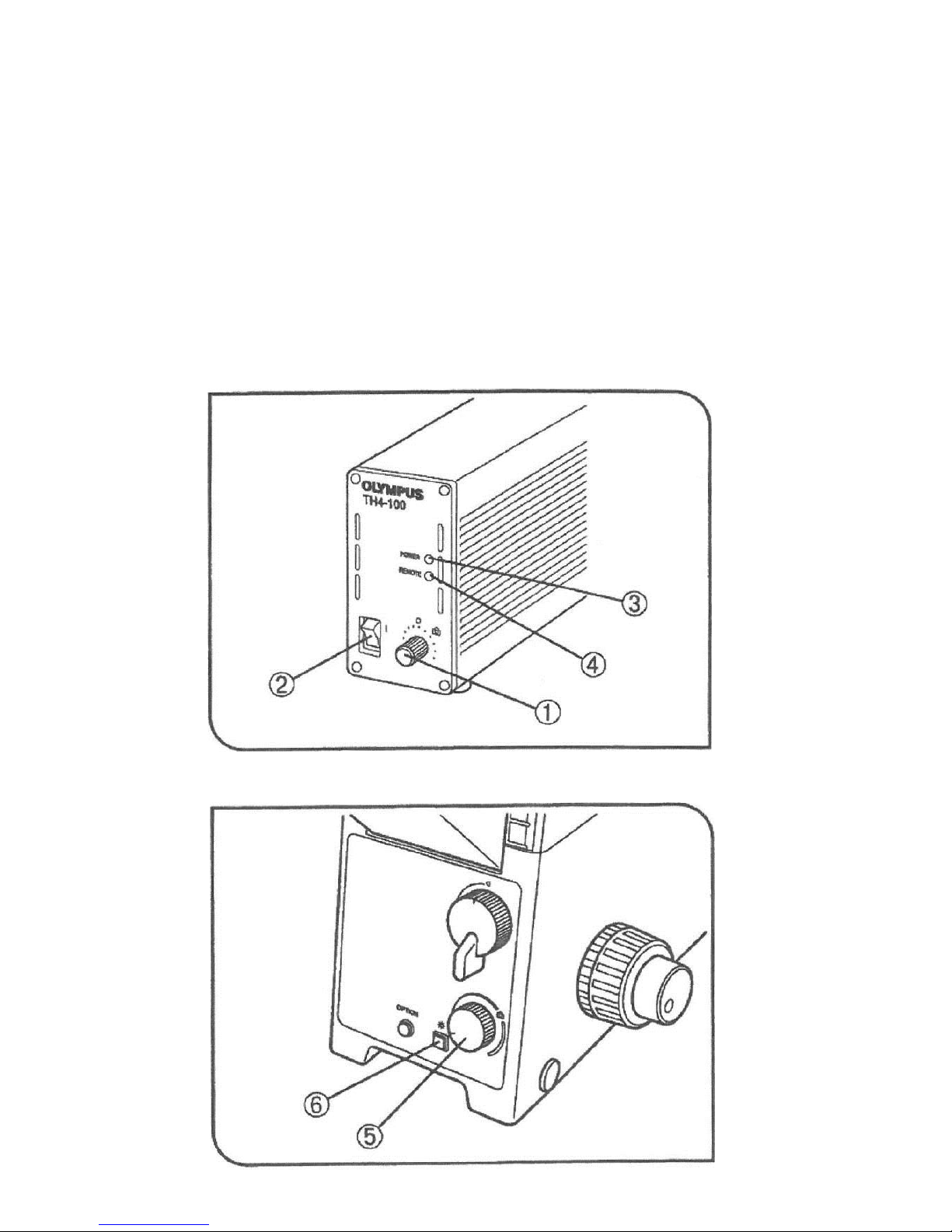
Olympus 1X71 User manual
Other Olympus Microscope manuals

Olympus
Olympus fsx100 User manual

Olympus
Olympus BH2-5RE Installation instructions

Olympus
Olympus CX41 Installation guide

Olympus
Olympus BX3-SSU User manual

Olympus
Olympus CHA-P User manual

Olympus
Olympus Pom User manual

Olympus
Olympus BX60 User manual

Olympus
Olympus X-Tr Operating instructions

Olympus
Olympus PROVIS AX70 Installation guide

Olympus
Olympus IX70 User manual

Olympus
Olympus BX60M User manual

Olympus
Olympus CX22LED User manual

Olympus
Olympus VANOX Operating instructions

Olympus
Olympus BHA-P User manual

Olympus
Olympus SZX7 User manual
Olympus
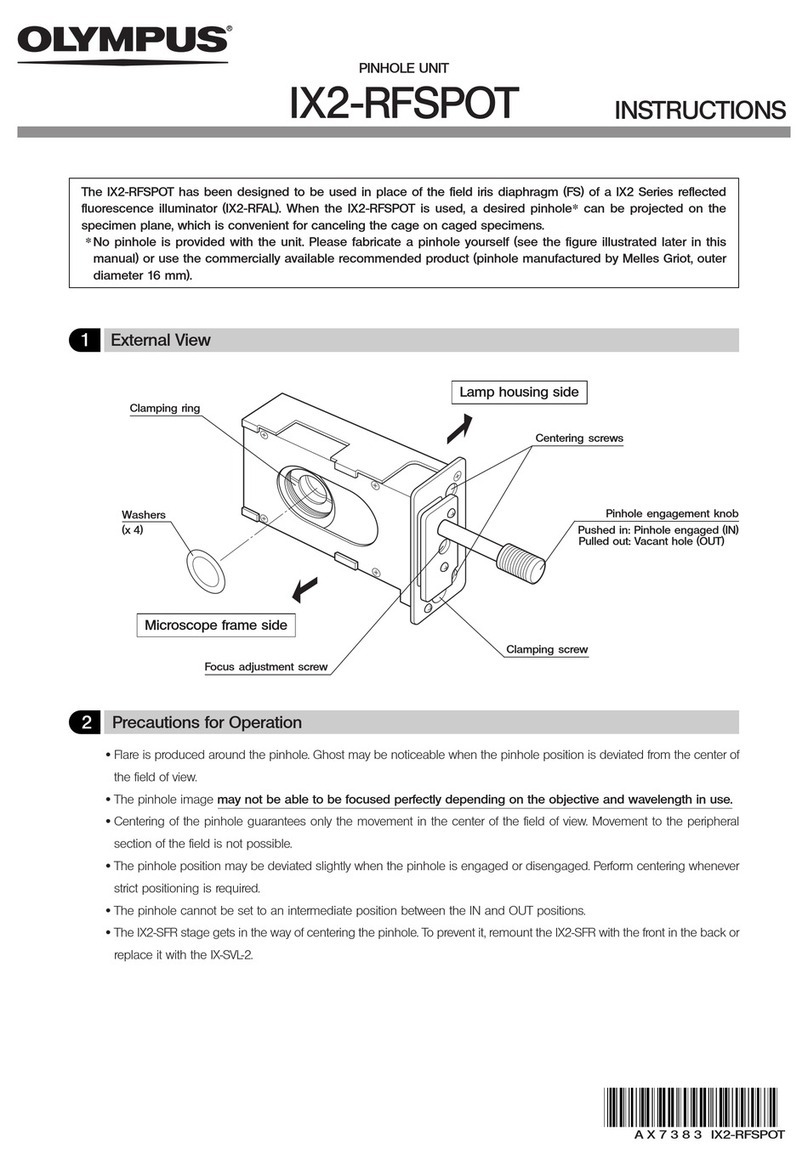
Olympus IX2-RFSPOT User manual

Olympus
Olympus BX-RLA2 User manual

Olympus
Olympus IX70 User manual

Olympus
Olympus DP22 User manual

Olympus
Olympus CH30 User manual