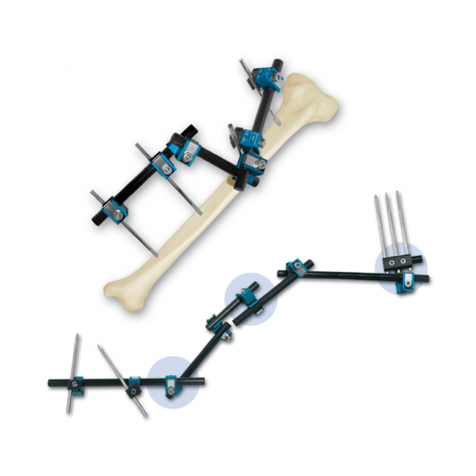
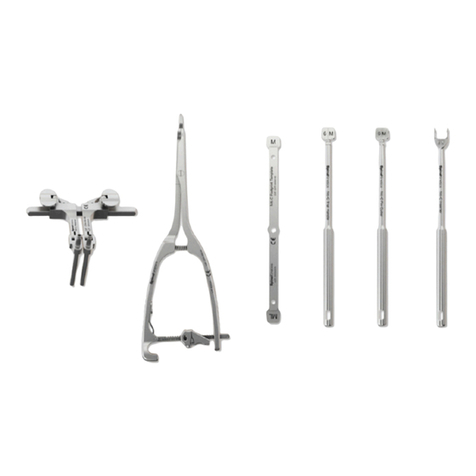
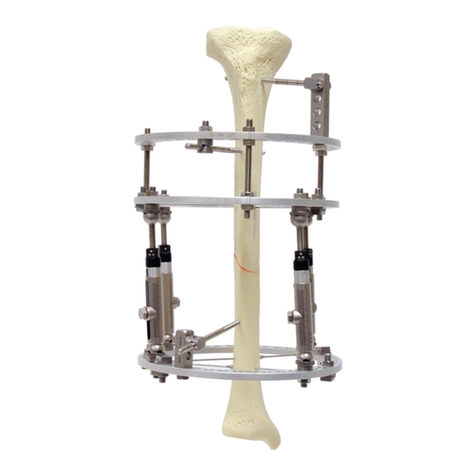
b) They should be inserted by pushing through the soft tissue and drilling through the bone; they should
never be drilled through soft tissue.
c) Wires should be inserted with awareness of the safe corridors to avoid damaging vital structures.
d) Used wire should always be disposed of if removed (since the tip can be blunted, and excessive heating of
the bone might occur).
e) To avoid causing injury the ends of wires should be protected with special covers or bent at the ends as
soon as they are tensioned.
To tension K-wires, the handles of the wire tensioner (54-1139) must be fully open and the arms fully inserted
over the wire down to the wire locking nut, ensuring that at least 6 cm of wire protrudes. When wires are
mounted on a circular ring, they must be tightened to a minimal tension of 1200 N. When wires with a central
olive are used, to stabilize a fragment, the tension must be reduced to 800/1000N. Grooved washers may be
used in numerous positions on a ring or distant from it. The tension possible varies with the position, up to a
maximum of 1000 Newtons. When it is attached directly to a ring, care must be taken to maintain the level of
tension, to avoid the wire becoming kinked and damaged.
POTENTIAL ADVERSE EFFECTS
Loosening, bending, rupture or fracture of the fixation device(s) or loss of fixation or migration which may
result in nerve, soft tissue, or organ damage, including perforation through the skin or other hemorrhaging.
Loss of anatomical position with non-union or malunions with rotation or angulation. Corrosion with
localized tissue reaction or pain. Infection, local or systemic. Pain, discomfort or abnormal sensations of
the nervous system resulting from the presence of the device. Cardiovascular disorders including venous
thrombosis, pulmonary embolism, or myocardial infarction. Bone loss or reduced bone density due to a
reduction in the tension applied to the bone.
1. Nerve or vascular damage following the insertion of wires or screws.
2. Deep or surface infections of the bone screw site, osteomyelitis, septic arthritis, including chronic
drainage of the bone screw sites following removal of the device.
3. Oedema or possible compartmental syndrome.
4. Joint constriction, subluxation, dislocation or loss of motor movement.
5. Premature bone callus consolidation during distraction.
6. Possible tension affecting the soft tissues and/or the fixation during manipulation of the callus (e.g.
corrections of deformities and/or elongation).
7. Lack of satisfactory bone regeneration, the development of mal-union or nonunion.
8. Fracture of regenerated bone, or at the bone screw holes, following removal of the device.
9. Loosening or breakage of the bone screws.
10. Bone damage due to erroneous bone screw selection.
11. Bone deformities or talipes equinus.
12. The persistence or recurrence of the initial condition subject to treatment.
13. New surgery to replace a component or the entire fixation frame.
14. Abnormal growth cartilage development in skeletally immature patients.
15. Foreign body reactions due to bone screws or components of the fixation frame.
16. Tissue necrosis secondary to the insertion of bone screws.
17. Pressure on the skin caused by external components when the free space is insufficient.
18. Limb dysmetria.
6