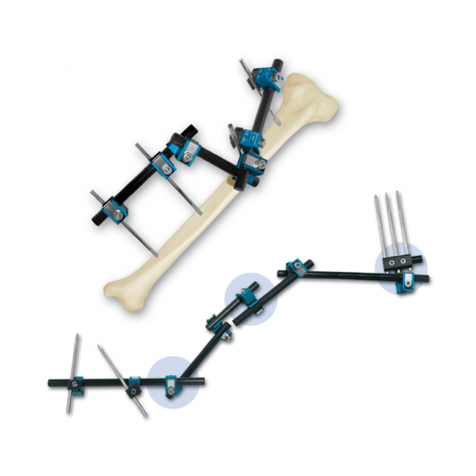
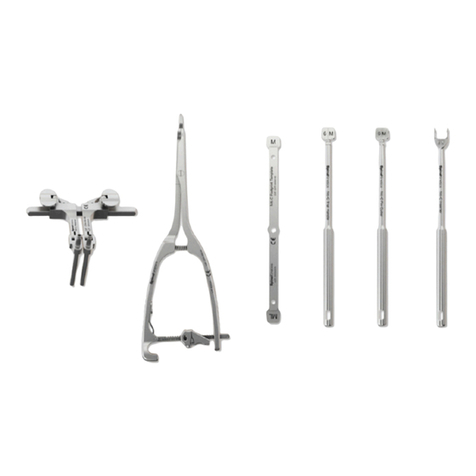
7
• When cleaning, fully submerge the instruments in the cleaning solution;
• Thoroughly scrub the single components in the cleaning solution with a soft brush until all visible soiling is removed. Metal brushes should be avoided. Clean
cannulations and holes using an appropriate brush ensuring that the full depth of the feature is reached;
• Rinse the single components in running tap water for at least 2 minutes. Ensure that running water passes through cannulations, and that blind holes are repeatedly
filled and emptied;
• Carefully hand-dry using absorbent, non-shedding cloth or industrial dryer for at least 5 minutes. Do not exceed 140°C (285°F).
Cleaning: Automated
• When the devices to be cleaned have lumens or present complexity, a preliminary manual cleaning may be required;
• Use a validated, properly maintained and calibrated washer disinfector;
• Place all the instruments into washer baskets.
• Place heavier devices in the bottom of the baskets.
• Connect cannulations to the proper injector jets.
• Wherever possible, all parts of disassembled devices should be kept together in one container.
• Orient instruments into the automated washer’s carriers as recommended by the washer manufacturer.
Orthofix recommends that cycle steps are at least as follows:
• Prewash at low temperatures;
• Main wash at 40-60°C for at least 5 minutes;
• Rinse with demineralized water;
• Thermal disinfection at 90-95°C for at least 1 minute;
• Rinse the components in sterile or freshly prepared purified water;
• Carefully hand-dry using absorbent, non-shedding cloth or industrial dryer. Do not exceed 140°C (285°F).
Maintenance, inspection and testing
• All instruments and product components must be visually inspected under good light for cleanliness. If some areas are not clearly visible, use a 3% hydrogen peroxide
solution to detect the presence of organic residuals. If blood is present, bubbling will be observed;
• All instruments and product components must be visually inspected for any signs of deterioration that may cause failure during use (such as cracks or damage to
surfaces) and functions tested before being sterilized. If a component or instrument is believed to be faulty, damaged or suspect, it must NOT BE USED;
• Inspect cables for wear and damage, ensuring that no cracks, tears, or other damage is found;
• Check to see probes have a continuous cutting edge and are free of nicks.
Packaging:
• Wrap the tray before sterilization with an approved sterilization wrap (a FDA-cleared sterilization wrap is recommended in US) or insert it into a rigid sterilization
container in order to prevent contamination after sterilization;
• Do not include additional systems or instruments in the sterilization tray. Sterility cannot be guaranteed if the sterilization tray is overloaded;
• The total weight of a wrapped instrument tray should not exceed 10 kg.
Sterilization:
• Steam sterilization is recommended. Gas plasma, dry heat and EtO sterilization must be avoided as they were not validated for Orthofix products;
• Sterilization must be at least two hours prior to use to allow the equipment to cool and stabilize;
• Handsets are NOT to be submerged in water to expedite cooling;
• DO NOT sterilize the generator or screen;
• Use a validated, properly maintained and calibrated steam sterilizer;
• The steam quality must be appropriate for the process to be effective;