0
12-03498C
-
'
In
troduction
Ma
-
(Accelerating
Voltage:
0
-
30
VDC)
In
1914,
J.
Franck and
G.
Hertz performed a landmark
experiment,providing important empirical support for
Max
Planck's quantum theory and for the model of the atom
suggestedby NielsBohr. Through the study of collisions
between electronsand gas molecules,Franck and Hertz
demonstratedthat energy
is
indeed quantized
in
atomic
interactions.For this work, they shared the1925Nobel Prize
in physics.
Because
of the importance of their experiment,
and because of itsconceptualsimplicity, it has become an
important experiment in the undergraduatelaboratory.
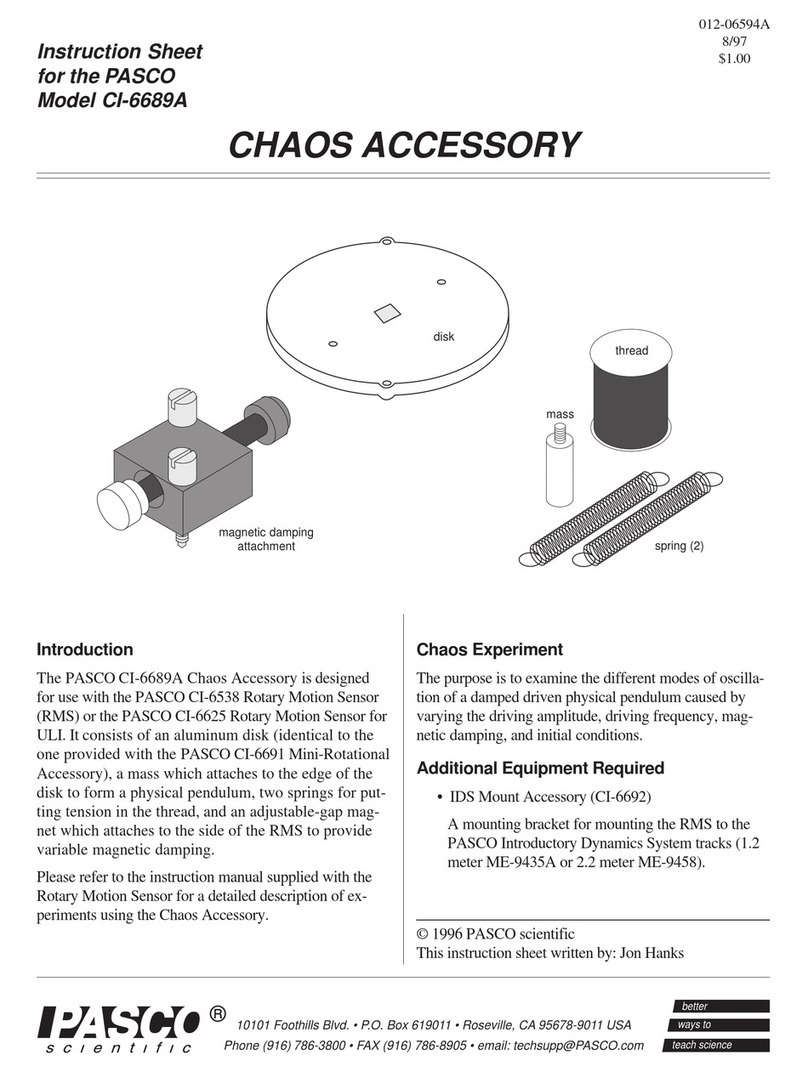
A
simplified
diagram
of the Franck
-
Hertz experiment
is
shown in Figure 1. In an oven
-
heated vacuum tube contain
-
ing mercury gas, electrons
are
emittedby a heated cathode,
and are then accelerated toward agrid that isat apotential
Va
relative
to
the cathode. Just beyond the grid isan anode,
which isat a slightly lower potential than thatof the grid (V
P
=
Va
-
AV;
AV
=
1.5
V;
where
V
is the anodepotential,and
Va
isthe grid potential,
).
If
the acceleratedelectronshave sufficientenergy when they
reach the grid,some of them will pass through and will reach
the anode. They will be measured
as
current Icby the
ammeter.
If
the electronsdon't have sufficientenergy when
they reach the grid,they will be slowed by
AV,
and will fall
back onto
the
grid.
Whether the electronshave sufficient energy
to
reach
the
anodedepends on three factors: the acceleratingpotential
(VJ,
the
opposingpotential
(AV),
and the nature of the
collisionsbetween the electrons and thegas molecules in the
tube.
As
long
as
the electron/moleculecollisionsare elastic,
the collector currentdepends only
on
Va
and
AV,
because
the electrons
lose
no energy
to
the gas. However, Franck and
Hertz discoveredthat Icwent through a seriesof maxima and
minima
as
theacceleratingpotentialwas varied. This implies
that the gasmolecules absorbenergy from
the
electronsonly
at specificelectronenergies(resonantenergies).
For example, the first excited state of mercury is4.9 eV
above the ground state.This is the minimum amount of
energy that the mercury atoms
can
absorbin collisionswith
the acceleratedelectrons. When
Va
is less than 4.9 V, the
electron/moleculecollisions
are
therefore elastic,
so
the
electrons loose no energy to the gas and arriveat the grid
with kineticenergy equal
to
eVa.
If
Va
>
AV,
the electrons
Rave sufficientenergy
to
overcome the opposing voltage,
and many of them will pass through the grid andreach the
anode, tobe measured
as
current.However, if
Va
equals4.9
V,
the electrons gain enoughkinetic energy to collide inelas-
tically with the mercury atomsjust as they reach the grid. In
these collisions, the mercury atoms absorb the
full
4.9 eV
P
-.
Anode
Grid
Cathode
ct
7""'
T
AV
(~1.5
V)
Ic
(AnodeCurrent:
0-5
PA)
Figure
1
SimplifiedDiagram
of
Franck
-
Hertz
Experiment
carriedby the electrons.The electrons no longer have suffi
-
cient energy to overcome
AV,
and they fallback onto the
grid. Ic
isthen a minimum.
As
Va
is
raised
beyond 4.9
V,
I,
increasesagain. However,
when
Va
reaches 9.8 V, the electrons
can
lose
all
their
energy
to
the gas molecules in two collisions.One collision
is likely
to
occur midway between the cathodeand the grid,
the other will occurjustas the electrons reach the grid.
Again, the electronsloose
all
their kinetic energy
in
the
inelastic collisions,
so
they fall back onto the grid, and Ic
again falls
to
a
minimum.
Because of multiple inelasticcolli
-
sionsbetween the acceleratedelectrons and the mercury
atoms,current minima are found whenever
Va
isa multiple
of 4.9
V.
Note:
The abovedescription is somewhat simpli-
fied.
Due
to
contactpotentials,the
total
energy
gain of the electronsisnot quite equal
to
eVa.
Therefore,
Va
will be somewhat higher than 4.9
V
when the firstcurrent minimum occurs. However,
the contactpotentials are a constantin the experi-
ment,
so
at successivecurrentminima
Va
will
alwaysbe a multiple of 4.9
V
higher than it was
when the firstcurrent minimum occured.