Revitive ProHealth 5572AQ User manual
Model number
5572AQ
Please read the
User’s Manual carefully
before using this product
USE ONLY AS DIRECTED
If symptoms persist, consult
your healthcare professional
ProHealth Drug-Free
Clinically Proven
Certified Medical Device
User’s Manual
CIRCULATION BOOSTER®
6289_IFU01_17694594.indd 16289_IFU01_17694594.indd 1 18/01/2021 13:3418/01/2021 13:34
2 3
What’s in the box? 4
Parts and Controls 5-6
Introduction to Revitive 7-8
Operator profile 7
Indications for use 8
Important Safeguards 9-13
Who should NOT use Revitive 9
Warnings and Cautions 10-12
Safety Precautions 13
Adverse Reactions 13
Instructions for use 14-29
Step 1: Setting up Revitive for use 14
Step 2: Using the SoTouch foot-pads 15-17
Step 3: Cleaning and storing 18
Troubleshooting 19-20
Technical Specifications 21-24
Warranty 25
Table of contents
How-to videos
For videos of setup and using your
Revitive system, go to:
support.revitive.com
For best results
Use daily
30-60 min
Intensity 40+
for 60+ days
2-year warranty
To activate your free 2-year warranty
please register your device at:
support.revitive.com
6289_IFU01_17694594.indd 2-36289_IFU01_17694594.indd 2-3 18/01/2021 13:3418/01/2021 13:34
4 5
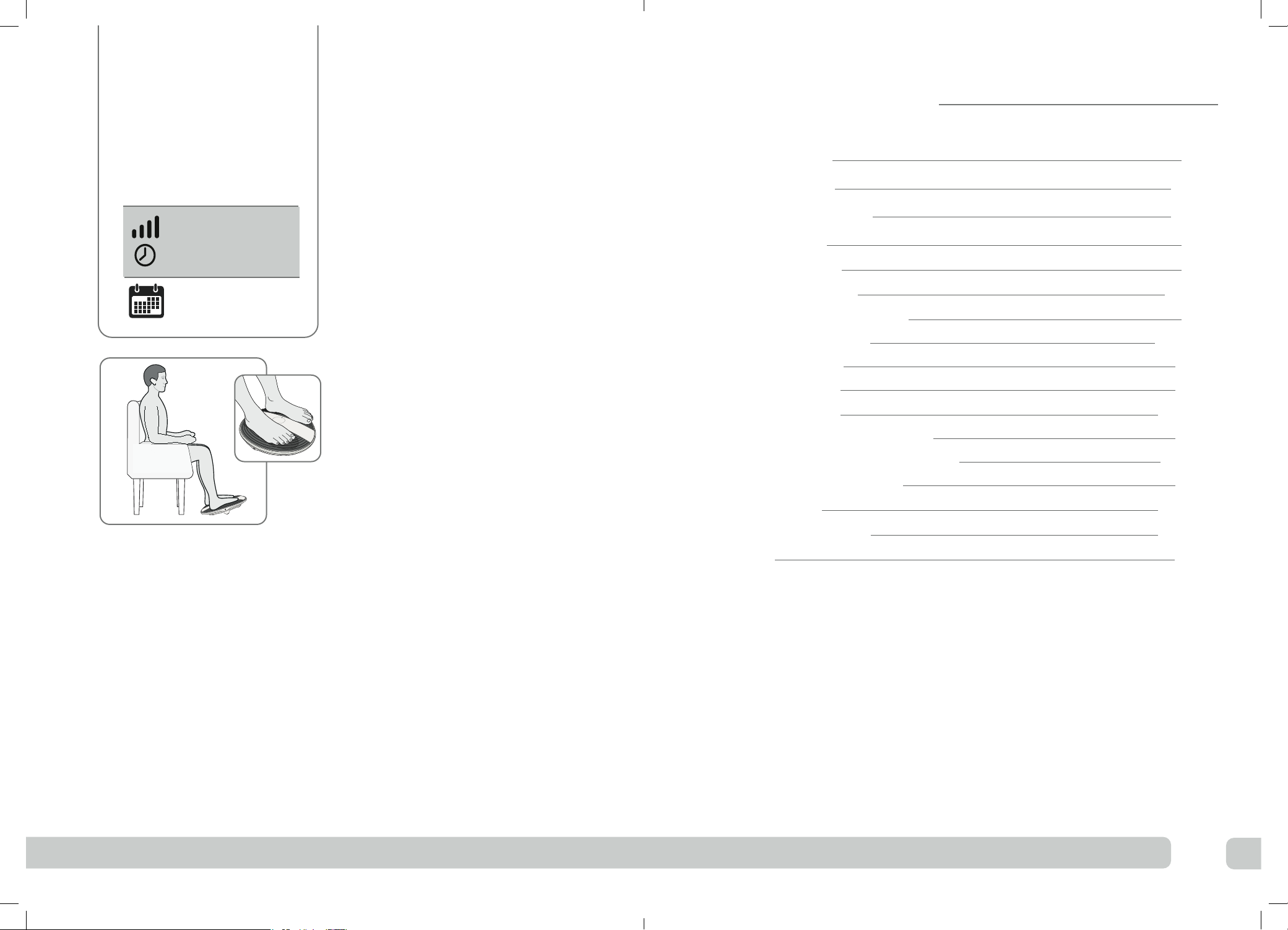
What’s in the box?
On opening the carton, please check that the following components are provided. If you
think anything is missing, please contact us using the helpline numbers on the back of
this booklet.
AC/DC power adaptor
Cable length 180cm
Revitive ProHealth
Remote control AAA batteries x 2
12
3 4
Control panel/
display
AC/DC power
adaptor socket
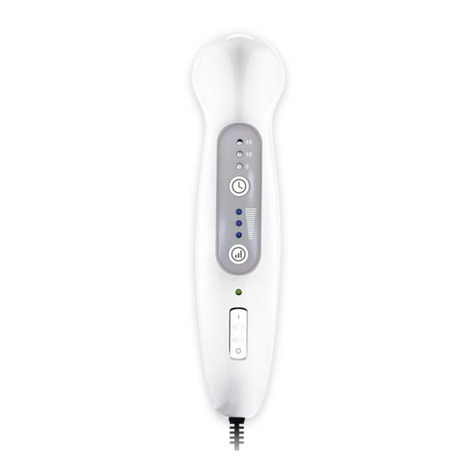
Parts and controls
SoTouch
Pads™
IsoRocker®
Power ON/OFF
Carry Handle
6289_IFU01_17694594.indd 4-56289_IFU01_17694594.indd 4-5 18/01/2021 13:3418/01/2021 13:34
6 7
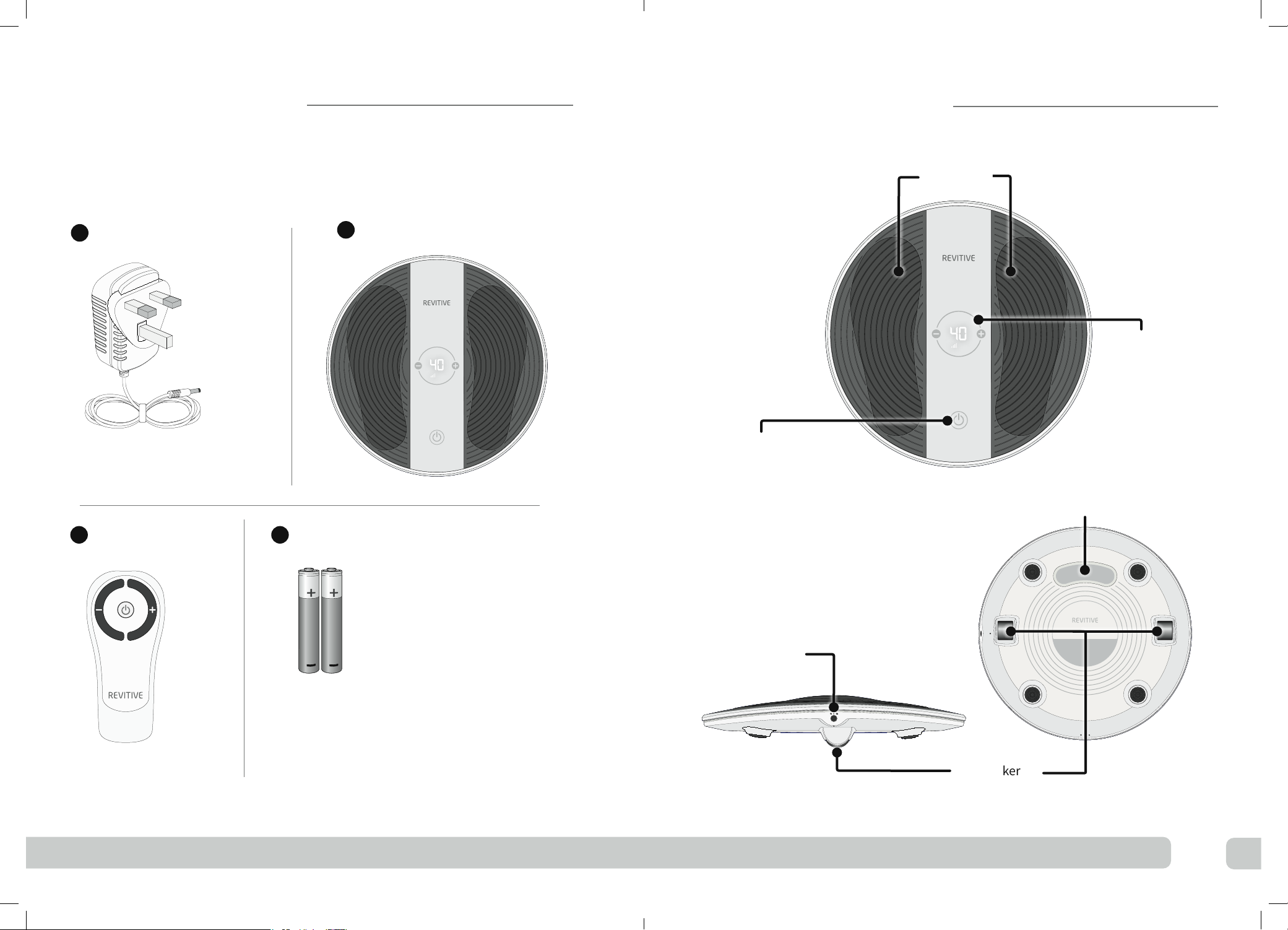
Power
ON/OFF
Remote control
Control panel
Intensity level
Intensity
Control – /+
Parts and controls
Time remaining
Intensity
Control – /+
Introduction to Revitive
Operator Prole
The intended operator profile is based on users in the home environment who may
suer from immobility, poor circulation and related issues such as pain, leg stiness
and swelling in the legs, ankles and feet.
Revitive is intended for use by the end user in a non-clinical setting and without the
supervision or intervention of a clinician during use.
A typical user may be of any adult age range and is not specific to any demographic of
gender, ethnicity or educational background.
6289_IFU01_17694594.indd 6-76289_IFU01_17694594.indd 6-7 18/01/2021 13:3418/01/2021 13:34

8 9
Read all instructions before use.
Save these instructions.
Revitive should not be used by some people.
Do not use if (contraindications):
• You are fitted with an electronic implanted device such as a heart
pacemaker or Automatic Implantable Cardioverter Defibrillator (AICD)
• You are pregnant
• You are being treated for, or have the symptoms of, an existing Deep Vein
Thrombosis (“DVT”)
Long periods of inactivity can put you at greater risk of developing Deep
Vein Thrombosis (DVT). DVT is a blood clot and usually occurs in a deep
leg vein. If part of the DVT breaks o it may lead to potentially life-
threatening complications such as pulmonary embolism.
If you have been inactive for prolonged periods and suspect you may
have a DVT, consult your doctor immediately. To prevent dislodging the
clot do not use Revitive.
In some cases of DVT there may be no symptoms. However it is important
to be aware of the symptoms that may include:
• Pain, swelling and tenderness in one of your legs (usually your calf)
• A heavy ache in the aected area
• Warm skin in the area of the clot
• Redness of your skin, particularly at the back of your leg, below
the knee
Consult your doctor as soon as possible if you show any signs of the
above symptoms.
Important safeguardsIntroduction to Revitive
Indications for use
When using the foot-pads, Revitive it is intended to:
• Help maintain leg vein health - by increasing circulation, delivering more oxygenated
blood and reducing swelling (oedema) in legs, feet and ankles.
• Improve circulation to reduce or prevent blood-pooling (stasis) - caused by poor
circulation (Chronic Venous Insuiciency/varicose veins)
• Reduce Pain and discomfort in the legs/ankles/feet - caused by poor circulation
(Peripheral Arterial Disease)
• Improve symptoms associated with varicose veins/ Chronic Venous Insuiciency
• Improve circulation in the legs - caused by Peripheral Arterial Disease
• Increase walking distance before the onset of claudication symptoms (pain) – caused
by Peripheral Arterial Disease
If you are otherwise healthy and have a sedentary lifestyle or spend long periods inactive,
Revitive may help to:
• Alleviate tired, aching & heavy legs, including cramp
• Reduce swollen feet & ankles
• Help maintain leg vein health
• Actively increase circulation
The above Indications for Use are certified under the Medical Devices Directive 93/42/EEC.
6289_IFU01_17694594.indd 8-96289_IFU01_17694594.indd 8-9 18/01/2021 13:3418/01/2021 13:34

10 11
Important safeguards
Warnings
When not to use Revitive:
• There are times you should not use Revitive. Do not use Revitive:
•In the presence of electronic monitoring equipment
•Together with a life-supporting medical electronic device
•When you are in the bath or shower
•While you are sleeping
• In conjunction with a brace or cast without first consulting with your doctor
• Use of this equipment adjacent to or stacked with other equipment should be
avoided because it could result in improper operation. If such use is necessary,
this equipment and the other equipment should be observed to verify that they
are operating normally
Additional Warnings:
• Use of cables other than those specified or provided by the manufacturer of this
equipment could result in increased electromagnetic emissions or decreased
electromagnetic immunity of this equipment and result in improper operation
• Portable RF communications equipment (including peripherals such as antenna
cables and external antennas) should be used no closer than 30 cm (12 inches)
to any part of the Revitive System, including cables specified by the manufacturer
Warnings
Consult with your doctor before using this device if:
• You are in the care of a doctor
• You have a history of heart problems
• You have had medical or physical treatment for your pain
• You have suspected or diagnosed heart disease
• You have suspected or diagnosed epilepsy
• You are unsure about the suitability of Revitive for you
• You are unsure about the cause of your symptoms
If you have a metallic implant:
• If you have a metallic implant, you may experience pain or discomfort near the
implant when applying electrical stimulation. If this should occur discontinue use
and seek advice from your doctor
• The electrical stimulation may feel more intense close to a metallic implant. It is
safe to continue use provided no pain is experienced. You may need to adjust the
intensity to a comfortable level
When applying foot-pads:
• Do not apply foot-pads directly on these areas:
•on open wounds or rashes; swollen, red, infected or inflamed areas; or skin
eruptions (such as phlebitis, thrombophlebitis, varicose veins, cellulitis)
•on, or close to, malignant tumours
•on areas treated with radiotherapy (within the past 6 months)
•near the thorax (chest) may increase the risk of cardiac fibrillation
•on your head, face, neck or chest
• Ensure that any moisturiser/gel/balm is evenly applied and thoroughly absorbed
into the skin before applying stimulation. There is a chance that uneven application
of a moisturiser/gel/balm could increase the risk of skin irritation or burn, when
using the electrical stimulation
• Symptoms may worsen during the initial treatment phase before getting better.
This may occur if the body has not fully adjusted to increased muscular activity
and blood circulation. If this occurs, reduce the intensity and the duration of
treatment which will reduce the initial symptoms. If symptoms persist consult
with your doctor
6289_IFU01_17694594.indd 10-116289_IFU01_17694594.indd 10-11 18/01/2021 13:3418/01/2021 13:34

12 13
Important safeguards
• Do not spill liquid on Revitive or its accessories
• Do not overload the electrical socket
• Keep device out of the reach of children
• Keep power cords and cables out of the reach of children to prevent risk of
strangulation
• Revitive foot-pads may be used by multiple persons. Ensure device is cleaned aer
each use
• During use, do not touch foot-pads with your hands until Revitive has powered o
• Use Revitive only with the accessories supplied by, or purchased from, the
manufacturer
• Check cables periodically for damage
• Do not open Revitive or repair it yourself. This will invalidate your warranty and
may cause serious harm
• In the unlikely event your Revitive malfunctions, disconnect it from the power
source and contact your nearest authorised agent
• Revitive has passed the required tests for Electromagnetic Interference (EMI); it
may still be aected by excessive emissions and/or may interfere with more
sensitive equipment
• It has been reported that some universal remote control devices (e.g. for TV etc.)
can change the settings on Revitive if used during a treatment. If this should occur,
simply adjust the time or intensity settings on Revitive back to where you want
them using the Revitive Remote Control, or using the device Control Panel
• Aer any exposure to hot or cold temperatures outside the specified operating
range of 10 – 40°C allow the product to re-adjust to the recommended operating
temperatures to ensure continued product performance
Adverse Reactions
• If you experience adverse reactions, stop using Revitive and consult with your
doctor immediately.
Cautions
When applying stimulation:
• Be careful when applying stimulation over areas of skin that lack normal
sensation.
It may cause skin irritation due to the inability to feel stimulation until the intensity
is too high. Use a low intensity to achieve a gentle muscle contraction, and/or
use for a shorter time, to avoid over-stimulation. Check for signs of skin irritation
(redness), bruising or pain. If in doubt consult your doctor
• This product is not intended for use by persons with reduced physical, sensory or
mental capabilities, unless they are supervised by a person responsible for their
safety
• Be careful when applying stimulation:
•Aer recent surgical procedures (within the past 6 months) as stimulation may
disrupt your healing process
•If your tissues are likely to bleed following an injury such as a muscle tear - it is
recommended not to stimulate the immediate area within the first 12 hours
aer sustaining the injury. Use a low intensity and/or shorter time to avoid
over-stimulation
•Aer a long period of immobility or inactivity – use a low intensity and shorter
time to avoid over-stimulation or muscle fatigue
Additional Cautions:
• The long term eects of electrical stimulation are unknown
• Electrical stimulation may not work for every user, please seek advice from
your doctor
Safety Precautions
•Do not stand on the machine. Use only when seated
• Do not position Revitive so that it is diicult to disconnect or turn o
• Use Revitive only for its intended purpose
• Do not expose Revitive to extreme heat
6289_IFU01_17694594.indd 12-136289_IFU01_17694594.indd 12-13 18/01/2021 13:3418/01/2021 13:34
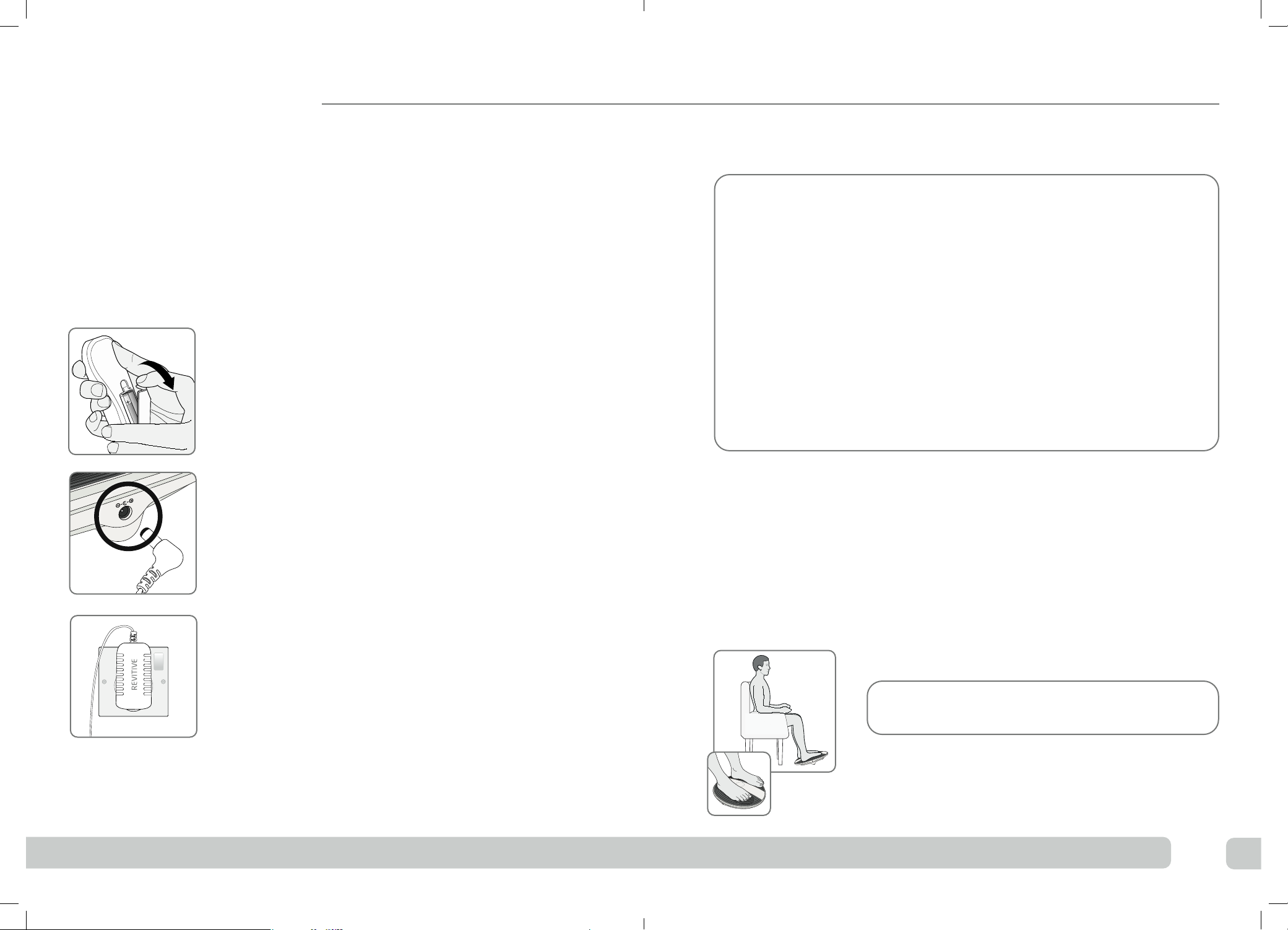
14 15
Instructions for use
Step 1: Setting up Revitive for use
aRemove all parts from the box:
• AC/DC power adaptor
• Revitive ProHealth device
• Remote control
• AAA batteries x 2
See “What’s in the box?” on page 4 to help identify the parts.
bLocate the remote control and insert the batteries.
cLocate the AC/DC power adaptor.
Plug the small end of the power cord into the socket
on the side of the Revitive.
dPlug the power adaptor into the nearest electrical
socket.
Before you use Revitive for the first time, read the Important Safeguards on pages
9-13. If in doubt, consult your doctor before using the product.
Step 2: Using the SoftTouch foot-pads
aIt is important that you are well hydrated. We recommend that you apply a
moisturiser to the soles of the feet to help hydrate the skin and improve the delivery
of electrical stimulation.
We also recommend drinking a glass of water before using Revitive.
For best results:
• Use Revitive foot-pads for at least 30 to 60 minutes each day, 7 days a week.
If you have varicose veins (CVI), use Revitive foot-pads for 60 minutes per day.
• It is important to use Revitive at a high enough intensity to give you a strong
comfortable muscle contraction. Most people achieve a strong muscle
contraction over intensity level 40 (intensity range 1-99).
• If you are diagnosed with a long term medical condition such as High
Blood Pressure, High Cholesterol, Diabetes, Osteoarthritis, Chronic Venous
Insuiciency (CVI), Peripheral Arterial Disease (PAD) or COPD, it can take up to
8 weeks to help reduce symptoms associated with these chronic conditions.
Do not use Revitive, using EMS stimulation, for more than 6 sessions of 30 minutes
(or the equivalent) per day.
Sit with
both bare feet
on the foot-pads
bGet into a comfortable seated position.
For best results sit with your knees at a 90 degree angle
(right angle).
cPlace Revitive on the floor in front of you.
Revitive is designed to be used while seated.
Never stand on the Revitive unit.
dPlace both bare feet on the foot-pads.
Make sure you remove all footwear, including socks/
stockings.
6289_IFU01_17694594.indd 14-156289_IFU01_17694594.indd 14-15 18/01/2021 13:3418/01/2021 13:34
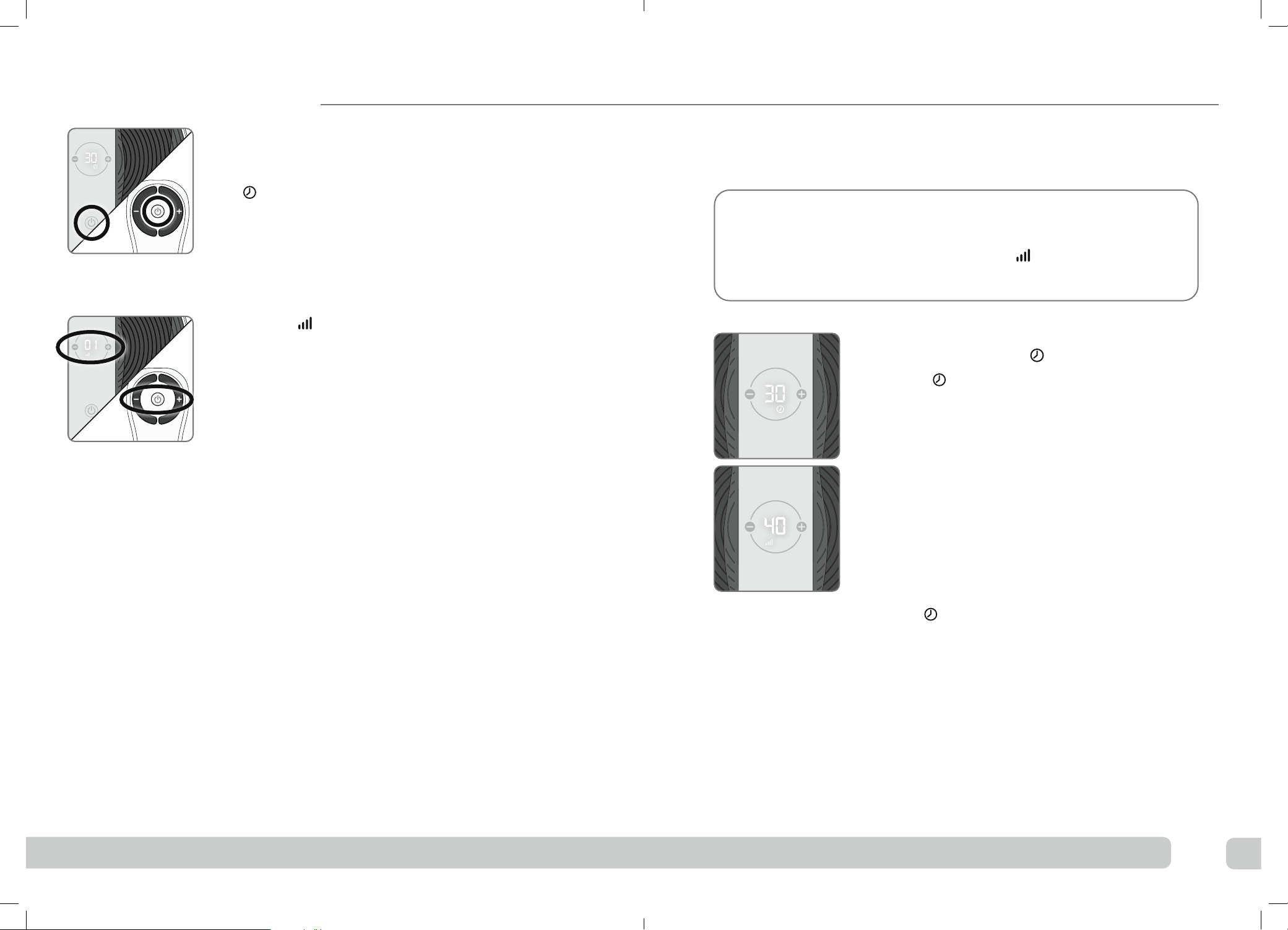
16 17
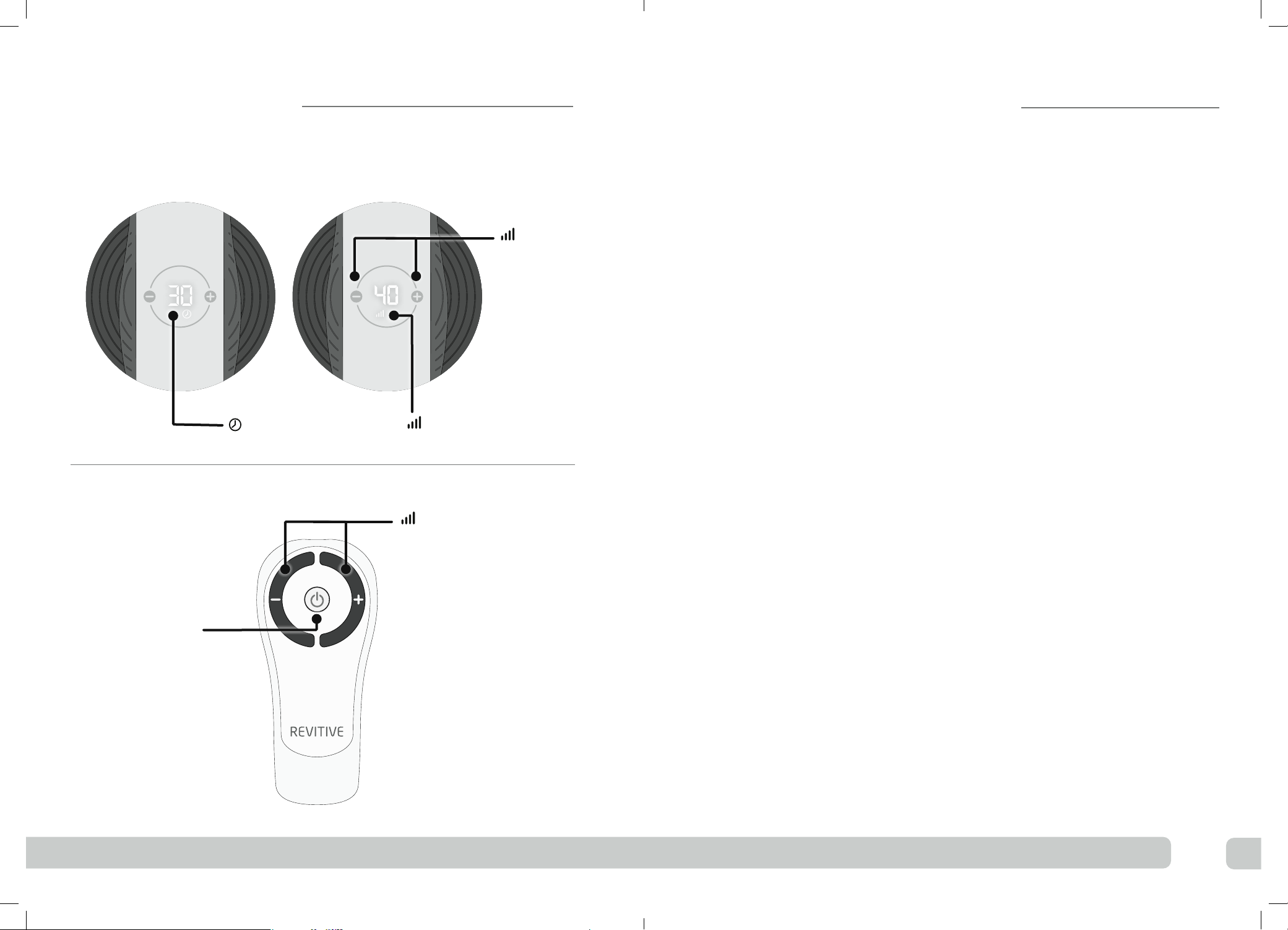
ePress the power button on the device or on the remote
control to turn Revitive on.
You will hear a beep and the display will read
30 minutes.
To turn o Revitive at any time during operation,
press the power button on the device or on the remote
control.
fPress the (+) Intensity Control one time to
start stimulation.
You can use the buttons on the control panel or
remote control. Ensure both feet are on the foot-
pads when increasing the intensity.
Press the (+) button to increase the level.
Press the (–) button to decrease the level.
Instructions for use
gEnsure you familiarise yourself with the feeling of the electrical muscle stimulation.
Use on a low intensity for 30-60 minutes once per day for a few days.
Set the intensity at a level where you can feel the stimulation in your feet which
creates a calf muscle contraction.
It is normal to feel tingling or varying sensations in your feet and calf muscles.
Revitive does not vibrate.
hAer a few days of familiarisation, use a higher intensity that provides you with
strong but comfortable calf muscle contractions.
Most people experience a strong contraction at intensity level 40 or over.
The IsoRocker® enables Revitive to rock up and down once muscle contractions are
nearing the desired level. The rocking is caused by muscle contractions in the leg,
and the resulting ankle movement allowed by the IsoRocker is a good indicator
that the intensity level is suicient to provide the therapy. Increasing the intensity
above this level will enhance the therapy, but should not be allowed to become
uncomfortable.
If you feel pain or discomfort:
• Remove one or both feet from the foot-pads to stop the stimulation
• Lower the intensity of the foot-pads using the (–) Intensity Control on
the control panel, or remote control, before replacing your feet on Revitive
iWhen you have finished setting the intensity, the
display reverts to the Time Display.
The Time Display will count down will count
down from 30 minutes as Revitive cycles through
its program.
Each time you adjust the intensity, the intensity level
shows in the display. Again, when you have finished
adjusting the intensity, the display reverts to the
Minutes-counter, continuing to count down the
session.
jWhen Revitive times out, the Time Display reads 00 and you will hear
three beeps.
kRevitive switches itself o automatically.
70
3o
You can set the intensity between 1-99.
Please note that the intensity required may vary from day to day.
6289_IFU01_17694594.indd 16-176289_IFU01_17694594.indd 16-17 18/01/2021 13:3418/01/2021 13:34
18 19
Step 3: Cleaning and storing Revitive
aEnsure Revitive is switched o.
bWipe down the foot-pads with a so damp cloth.
Do not clean with chemicals.
Do not immerse Revitive in water.
cStore Revitive in a cool, dry and dust-free location.
Store out of direct sunlight.
Replacing the Batteries
The remote control requires two AAA 1.5V batteries to operate.
Troubleshooting
Problem Possible Cause Solution
Revitive is on (lights
illuminated on the
LED display) but
I cannot feel the
electrical stimulation
through the foot-
pads.
Not placing both bare feet
on the foot-pads at the
same time.
Ensure that your feet are bare and each
foot is placed on each of the foot-pads
at the same time. Keep increasing the
intensity up to a maximum of 99 until
you feel the stimulation.
Your feet may be dry. Moisturise the soles of your feet to
improve conductivity and stimulation
and try the procedure again. You may
also have to increase the intensity
level.
You may be dehydrated. Drink plenty of water before and aer
using the device. The device uses your
body to create the electrical circuit.
Water is an excellent conductor of
electricity and if your body is less
hydrated (below 60%) then the
stimulation may be less, therefore it
is important to always remain well
hydrated.
The intensity level may
be on too low a setting.
This is a very safe device. Keep
increasing the intensity level towards
99 until you feel the stimulation. You
may find that you have to increase the
intensity level as you get used to the
therapy. The aim is not to get to 99 but
to find a setting that produces strong
muscle contractions in your calves and
is comfortable for you.
If, having tried the solutions
above, you still cannot feel
the stimulation:
Test the device by placing one hand
across both foot-pads at the same time
(The heel end of the foot-pads is easiest).
With your other hand, and starting from
zero, increase the intensity level until
you can feel the stimulation. If you can
feel the stimulation through your hand
then the device is working. If on 99 you
still cannot feel the stimulation then
please contact your authorised dealer.
Instructions for use
6289_IFU01_17694594.indd 18-196289_IFU01_17694594.indd 18-19 18/01/2021 13:3418/01/2021 13:34
20 21
Problem Possible Cause Solution
No power or lights
to Revitive when it is
switched on.
AC Adaptor not switched on
at the electrical socket or AC
Adaptor not plugged into device
properly.
Check electrical socket power
is switched on and the AC Adaptor
is plugged into the device correctly.
If still not working – contact your
authorised dealer.
Revitive is not
vibrating. REVITIVE IS NOT DESIGNED TO VIBRATE.
The IsoRocker® is not
"rocking".
It is the muscles in your legs
that cause the "rocking", the
IsoRocker® is a pivot.
The IsoRocker® will only rock
when increased to an intensity
which causes suicient calf muscle
contraction. It may be that you
cannot comfortably increase the
intensity high enough until you
get used to the sensation. It is
important that intensity is adjusted
to a level that is comfortable.
The device is too far in front
of you.
Sit with your knees at a 90 degree
angle.
When using the
IsoRocker® on a
hard floor it makes a
tapping noise.
Incorrect positioning of the
device or too high an intensity
level.
Adjust the positioning of the
device or lower the intensity level
to reduce the device tapping.
Alternatively use a floor mat under
the device to cushion the sound.
My legs are aching
aer treatment.
You may have the
intensity
on too high a setting and your
muscles are being overworked.
Leave adequate time aer each
treatment to allow the muscles
to recover (just like aer vigorous
exercise!). On your next session
start on a lower setting (where
you can feel the mild electrical
stimulation and it is comfortable)
and reduce the duration until your
muscles have acclimatised to the
stimulation.
For more information, including Frequently Asked Questions,
please visit support.revitive.com
Technical Specications
Name of product Revitive ProHealth
Model 5572AQ
Frequency (+/- 10%) 20Hz – 44Hz
Output current Max 15mA
Weight
1.12 Kg +/- 0.5Kg
Dimensions 360mm (Ø) x 70.9mm (D)
Power consumption 5W
AC adaptor
CE Approved
Power source
Input (adaptor used)
Output
100-240V AC ( ), 50/60Hz, 0.18A
5V ( ) DC ,1.0A
Applied parts
Parts of Revitive that in normal use come into
physical contact with the user.
SoTouch Pads (Foot-Pads) - 195cm2
Durable Period (Service Life) of Device and
Remote control 4 years
The Remote Control replicates the controls found on the device
Operating Frequencies 38 KHz
Operating Range Distance
0 to 25m at horizontal
0 to 18m at +/- 30 degree angle
from the horizontal.
Troubleshooting
6289_IFU01_17694594.indd 20-216289_IFU01_17694594.indd 20-21 18/01/2021 13:3418/01/2021 13:34
22 23
Output Specifications for Electrical Muscle Stimulation (EMS):
Waveform Biphasic
Shape Square symmetrical with polarity reversal
Maximum Output Voltage (+/-15%) @500Ω 35Vp
Pulse Duration (+/-10%) 450-970µs
Net Charge @ 500Ω [0.001]mC
Maximum Power Density @ 500Ω 0.68 mW/cm²
ON Time (+/-10%) 1.90 - 8.30s
OFF Time (+/-10%) 1.00 - 1.50s
The values of PULSE DURATIONS, PULSE repetition frequencies and amplitudes, including
any d.c. component, shall not deviate by more than ± 20 % when measured with a load
resistance within the range specified.
If confirmation is required that the Revitive works within its Essential Performance aer a
certain period of time, contact the manufacturer
Complies with European Medical Devices Directive (93/42/EEC)
Device Lot number including year (YYYY) and month (MM) of
manufacture can be found on the box and back of unit
Item number
Contraindications, Warnings and Cautions
Make sure you understand these before using Revitive
Power
Time Remaining
Intensity Level
Center Positive Polarity
Class II medical electrical equipment double insulated
Type BF medical electrical equipment
Legal manufacturer of the device
EU/EC European Authorised Representative
Consult instructions for use
The Waste Electrical and Electronic Equipment Directive
(WEEE Directive).
At the end of the product lifecycle, do not throw this product
into normal household garbage, but take it to a collection point for the
recycling of electronic equipment
#YYYYMMXXXXX
LOT
REF
Symbols
Technical Specications
6289_IFU01_17694594.indd 22-236289_IFU01_17694594.indd 22-23 18/01/2021 13:3418/01/2021 13:34
24 25
UK Conformity Assessed
Product conforms to all applicable U.K. legislative requirements.
FCC mark
Certification mark employed on electronic products manufactured
or sold in the United States which certifies that the electromagnetic
interference from Revitive is under limits approved by the Federal
Communications Commission
Ingress Protection Rating
Use-by date YYYY MM DD
Humidity, temperature and air pressure limit for storage
and transport
Humidity, temperature and air pressure limit for operating
conditions
Aer any exposure to hot or cold temperatures outside the specified
operating range of 10 - 40°C allow the product to re-adjust to the
recommended operating temperatures to ensure continued product
performance.
Indoor Use Only
Medical device does not contain natural rubber latex
Do not disassemble
-20°C
20%
70°C
90%
500 hPa
1060 hPa
10°C
30%
40°C
75%
700 hPa
1060 hPa
Your 2-year warranty
To activate your free 2-year warranty please register your
device at: support.revitive.com
It is important to retain the retailer’s receipt
as proof of purchase. Staple your receipt to
this back cover for future reference.
Please quote the following information if the
product develops a fault. These numbers
can be found on the base of the product:
Model no: ......................................................
Lot no: ...........................................................
All Revitive devices are individually tested
before leaving the factory. In the unlikely
event of any device proving to be faulty
within 30 days of purchase, it should be
returned to the place of purchase for it to be
replaced.
If the fault develops aer 30 days and within
24 months of original purchase, you should
contact your local distributor quoting model
number and LOT number on the product, or
write to your local distributor at the address
shown.
You will be asked to return the product (in
secure, adequate packaging) to the address
shown with a copy of proof of purchase.
Subject to the exclusions set out below (see
Exclusions) the faulty device will then be
repaired or replaced and dispatched, usually
within 14 working days of receipt.
If, for any reason, this item is replaced during
the 2-year guarantee period, the guarantee
on the new item will be calculated from the
original purchase date. Therefore, it is vital
to retain your original till receipt or invoice
to indicate the date of initial purchase.
To qualify for the 2-year guarantee, the
device must have been used according to the
manufacturer’s instructions supplied.
Exclusions
Actegy, manufacturer of Revitive devices,
shall not be liable to replace the goods under
the terms of the guarantee where:
• The fault has been caused or is
attributable to accidental use, misuse,
negligent use or used contrary to the
manufacturer’s recommendations or
where the fault has been caused by
power surges or damage caused in
transit.
• The device has been used on a voltage
supply other than that stated on the
product or used with a power adaptor
other than the one supplied with the
product.
• Repairs have been attempted by persons
other than our service sta
(or authorised dealer).
• The device has been used for hire
purposes or non-domestic use.
• The device is second hand.
2Actegy are not liable to carry out any type of
servicing work, under the guarantee.
3Accessories such as electrode pads and bags
are not covered by the guarantee.
4Remote control batteries and any damage
from leakage are not covered by the
guarantee.
5This guarantee does not confer any rights
other than those expressly set out above and
does not cover any claims for consequential
loss or damage. This guarantee is oered
as an additional benefit and does not aect
your statutory rights as a consumer.
Technical Specications
Symbols
6289_IFU01_17694594.indd 24-256289_IFU01_17694594.indd 24-25 18/01/2021 13:3418/01/2021 13:34

26 27
6289_IFU01_17694594.indd 26-276289_IFU01_17694594.indd 26-27 18/01/2021 13:3418/01/2021 13:34
6289_IFU01_17694594 01.2021
Actegy Ltd
1 West Point
Western Road
Bracknell, RG12 1HJ
United Kingdom
Tel: +44 (0)845 871 5989
Distributor
UK:
1 West Point
Western Road
Bracknell, RG12 1HJ
United Kingdom
Tel: +44 (0)800 014 6377
info.uk@actegy.com
MDSS
Schigraben 41
30175 Hannover, Germany.
Copyright © 2021 Actegy Ltd. All rights reserved. Actegy®, Revitive®, Revitive ProHealth®,
Circulation Booster®, OxyWave®, IsoRocker® and SoTouch Pads™ are trademarks or
registered trademarks of Actegy Ltd. The Actegy product is a proprietary design and is
protected by applicable design laws.
CIRCULATION BOOSTER®www.revitive.com
ProHealth
6289_IFU01_17694594.indd 286289_IFU01_17694594.indd 28 18/01/2021 13:3418/01/2021 13:34
Table of contents
Other Revitive Medical Equipment manuals

Revitive
Revitive Medic Knee User manual
Revitive
Revitive UT1033 User manual

Revitive
Revitive Advanced Setup guide

Revitive
Revitive Medic 2837AB User manual
Revitive
Revitive Medic Knee User manual

Revitive
Revitive ProHealth User manual

Revitive
Revitive Medic Coach User manual

Revitive
Revitive Medic 5573AQ User manual
Popular Medical Equipment manuals by other brands

Getinge
Getinge Arjohuntleigh Nimbus 3 Professional Instructions for use

Mettler Electronics
Mettler Electronics Sonicator 730 Maintenance manual

Pressalit Care
Pressalit Care R1100 Mounting instruction

Denas MS
Denas MS DENAS-T operating manual

bort medical
bort medical ActiveColor quick guide

AccuVein
AccuVein AV400 user manual











