67
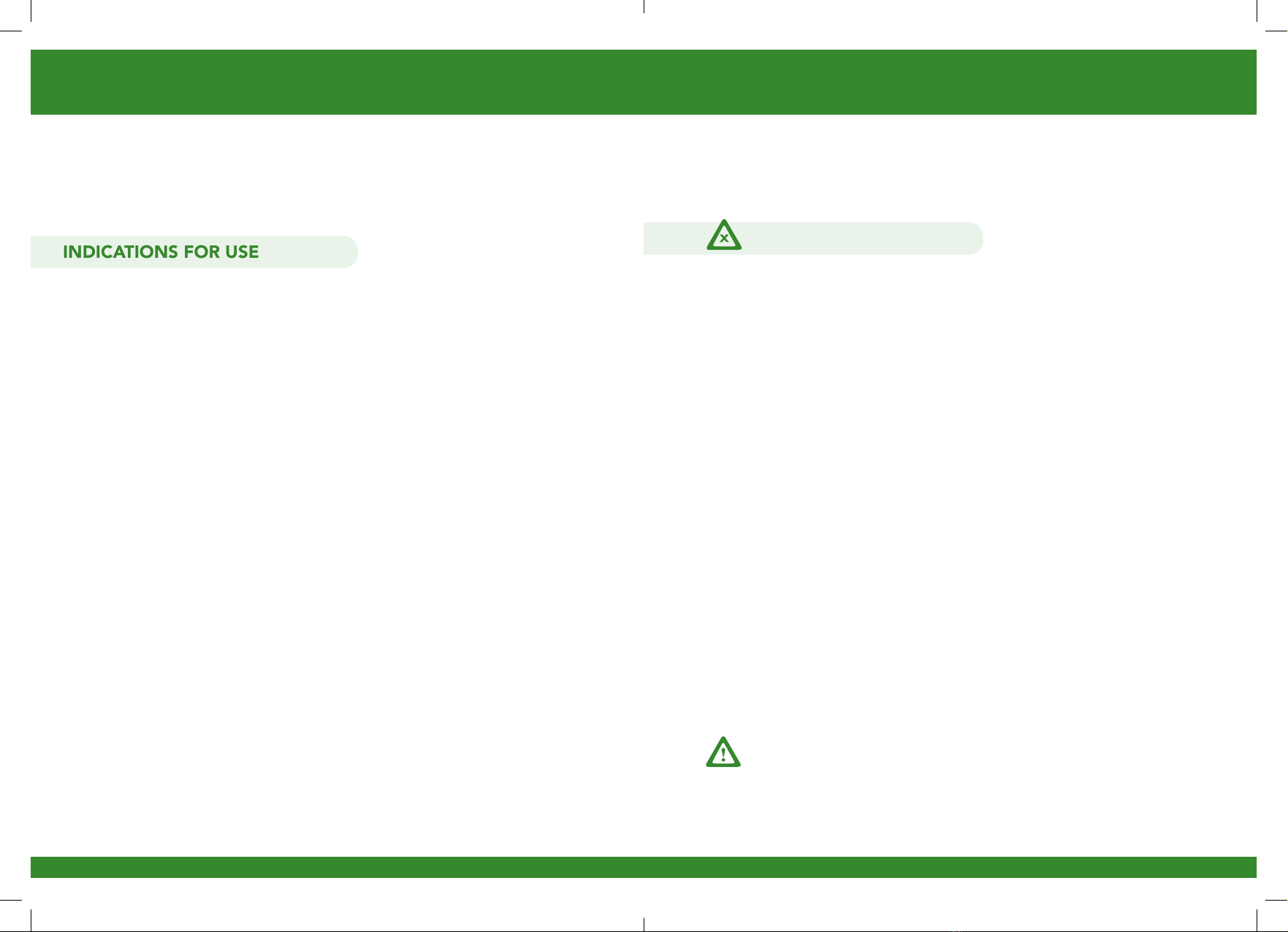
Before operating your REVITIVE Ultrasound device, please read
these instructions in full and save this manual for future reference.
• May help to relieve pain
• May help to aid healing •Used for the treatment of malignancy (application over a suspected or
confirmed tumour)
•Used on the skin over electronic implants, including pacemakers or
defibrillators
•Used on an infected or bleeding area, including tuberculosis
•Used on the skin over vascular (blood vessel) abnormalities (such as
haemangioma, capillary, lymphatic, arterial or arterio-venous
malformations)
•Used directly on the abdomen or lower back of pregnant women
•Applied directly over active epiphysis regions (growth plates), in the
presence of myositis ossificans (bone formed within the muscle) or
over the eyes, skull or reproductive organs
•Used over open wounds or fragile or damaged skin eg eczema
•Used on the front of the neck over the carotid sinus
•Used over spinal abnormalities e.g. spina bifida, following laminectomy
•Used over active deep vein thrombosis or thrombophlebitis
•Used on recently irradiated tissue (within 6 months)
•Used on heart, eye, testes, near brain, cervical ganglia, spine,
laminectomy sites (can cause spinal cord bleeding).
•Used by individuals who do not comprehend the instructions for
application
REVITIVE Ultrasound should not be:
If you have any doubts about the suitability of REVITIVE Ultrasound,
please consult your healthcare professional before using this product
Introduction to REVITIVE IMPORTANT SAFEGUARDS
READ ALL INSTRUCTIONS BEFORE USE
Suitable for people suffering from muscular injuries, aches, pains and
strains. Especially effective on lower back and shoulders.
INDICATIONS FOR USE CONTRAINDICATIONS
3269_IFU02_14269060.indd 6-7 24/07/2017 11:59