Sentec IPV 1 User manual

IPV® 1 System
Instruction Manual

II
©2023 Percussionaire® Corporation
ALL RIGHTS RESERVED
Percussionaire®, IPV®, TRUE-IPV® and Phasitron® are registered trademarks.
The IPV® 1 may be covered by one or more patents.
The contents of this document may not be reproduced in any form or communicated to
any third party without the prior written consent of Percussionaire® or Sentec. While every
effort is made to ensure the correctness of the information provided in this document,
neither Percussionaire® nor Sentec assumes no responsibility for errors or omissions. This
document is subject to change without notice.
This manual was originally released and supplied in English. For a list of available
replaced at any time. Ensure this manual is the most current applicable version. To obtain
the most recent version, contact Sentec Customerservice.us@sentec.com or visit Sentec.
com.
CAUTION: Federal law (U.S.) restricts this device to sale by or on the order of a
physician.

III
Table of Contents
1: Introduction..................................................................................................................................................1
About This Instruction Manual ....................................................................................................................1
Related Documents and Resources ..........................................................................................................1
Glossary of Symbols .........................................................................................................................................2
Safety Information ............................................................................................................................................3
Warnings..........................................................................................................................................................4
Precautions .....................................................................................................................................................5
Technical Assistance.........................................................................................................................................5
2: Intended Use................................................................................................................................................6
Intended Purpose..............................................................................................................................................6
Intended Use Environment ...........................................................................................................................6
Intended User Profile .......................................................................................................................................6
Indications for Use ............................................................................................................................................6
Expected Clinical Benefits of IPV®...............................................................................................................6
Contraindications..............................................................................................................................................7
3: Principles of Operation...........................................................................................................................8
4: Description....................................................................................................................................................9
IPV®1 System ......................................................................................................................................................9
Front Panel ...........................................................................................................................................................9
Control Functions ..........................................................................................................................................10
Back Panel..........................................................................................................................................................11
Blended Gas/Air Connection.....................................................................................................................11
5: Digital Display .........................................................................................................................................12
POST (Power-On Self-Test)..........................................................................................................................12
Wake Mode .......................................................................................................................................................13
Active Mode......................................................................................................................................................13
Report Mode ....................................................................................................................................................14
Sleep Mode.......................................................................................................................................................14
Fault Mode ........................................................................................................................................................15
Fault Detection................................................................................................................................................15
Fault Logging ...................................................................................................................................................15
Digital Display - Setup .................................................................................................................................16
6: Setup .............................................................................................................................................................17
Roll Stand Assembly......................................................................................................................................17
Attaching IPV®1 to Stand...................................................................................................................18
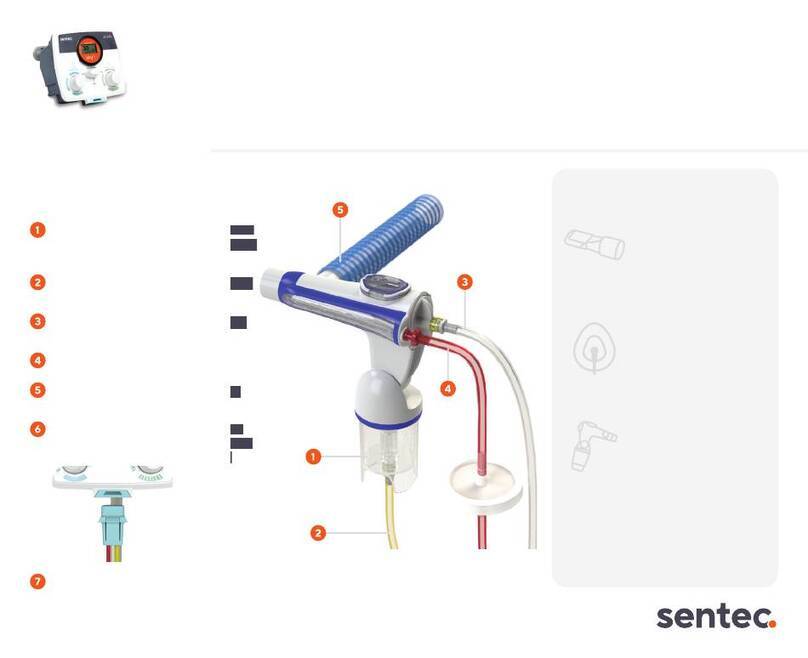
Breathing Circuit - Phasitron®5 UC.........................................................................................................19
Breathing Circuit - Phasitron® 5 UC - Components ..................................................................20
Breathing Circuit - Phasitron® 5 UC - Configurations........................................................................20
Breathing Circuit - Connecting to the IPV®1.............................................................................21
Connecting Tubing to Phasitron® 5 ...............................................................................................21
Adding Liquid Solution ......................................................................................................................22
7: Pre-Use Check...........................................................................................................................................23

IV
8: Prepare for Patient-Airway Connection .....................................................................................26
9: Administer IPV®Therapy ....................................................................................................................26
10: Cleaning and Maintenance Protocol ........................................................................................28
Digital Display ........................................................................................................................................28
Stand Assembly......................................................................................................................................28
Breathing Circuit - Phasitron® 5 UC ................................................................................................29
Phasitron® 5 UC Breathing Circuit Cleaning Process...............................................................30
11: Troubleshooting...................................................................................................................................31
12: Service........................................................................................................................................................31
IPV®1 Device ...........................................................................................................................................31
Stand ..........................................................................................................................................................31
Breathing Circuit - Phasitron® 5 UC ................................................................................................31
Service........................................................................................................................................................32
Changing Display Module Batteries..............................................................................................32
Disposal of Equipment........................................................................................................................32
13: Limited Warranty..................................................................................................................................33
Warranty Exclusions and System Performance.........................................................................33
14: Technical Specications....................................................................................................................34
15: Glossary ............................................................................................................................................ 35

1
1: Introduction
The IPV® 1 system is intended for patients needing an airway clearance therapy for
mobilization of secretions, lung expansion therapy, or the treatment and prevention of
pulmonary atelectasis.
The IPV® 1 system is designed specifically for non-continuous institutional/hospital
use to provide airway clearance and lung recruitment therapy. The system comprises
a controller unit and breathing circuit called a Phasitron® 5. This circuit features a
unique sliding venturi mechanism that provides a lung protective strategy while
delivering high-frequency pulses, augmenting diffusive gas exchange throughout the
lungs, promoting airway clearance and lung expansion.
About This Instruction Manual
Related Documents and Resources
This manual contains information for operating the IPV® 1 therapy system. Before
operating the IPV® 1, the user must thoroughly read and understand these instructions
for use.
It is the user’s responsibility to follow the instructions given and to keep the
instructions for use near the device to ensure correct operation. If the safety
instructions are not followed, the patient may be at risk.
This section contains the following:
• Related documents and additional resources
• Symbol definitions
• Safety information, including warnings and cautions
• Technical assistance information
The current version of this manual, specifications, clinical studies, and additional
information is available at:
Customerservice[email protected]
Other manuals for IPV 1
1
Table of contents
Other Sentec Medical Equipment manuals
Popular Medical Equipment manuals by other brands

Getinge
Getinge Arjohuntleigh Nimbus 3 Professional Instructions for use

Mettler Electronics
Mettler Electronics Sonicator 730 Maintenance manual

Pressalit Care
Pressalit Care R1100 Mounting instruction

Denas MS
Denas MS DENAS-T operating manual

bort medical
bort medical ActiveColor quick guide

AccuVein
AccuVein AV400 user manual