Sentec LuMon User manual

fortheLuMon System
2ST200-110Rev013│2023-02
For the LuMon System
GUIsoftware1.0.x.x/TICsoftware1.6.x.xxx
LuMon Belts/SensorBelt

2of 93
fortheLuMon System
Warranty
The manufacturer warrants to the initial purchaser that each new component of the LuMon System will be
free from defects in wor
itsownchoicerepairorreplaceanycomponent forwhich themanufactureracknowledgesthewarrantycover
withareplacementcomponent.
Warranty exclusions and system performance
Sentec AG can neither guarantee or verify instrument performance characteristics nor accept warranty claims
or product liability claims if the recommended procedures are not carried out, if the product has been subject
tomisuse,neglectoraccident,ifthe producthasbeendamagedbyextraneouscauses,ifaccessoriesotherthan
thoserecommendedbySentec AGareused, ifthewarrantysealonthe lowersideofthe monitorisbroken, orif
instrumentrepairsarenotcarriedoutby
SentecAuthorizedLuMon Technicians
.
Design/patents related to the LuMon System: Please refer to the specification sheets for the LuMon
System:www.Sentec.com/education/eit/plpm-eit
Trademarks: Sentec , Advancing Noninvasive Patient Monitoring , LuMon and Sentec EIT are trademarks
ofSentecAG.
Terms of use of software components: Sentec devices that use software may use Sentec, third party and/or
open-source software, depending on their setup. Sentec, third party and/or open-source software may be
subject to different terms of license. Respective information regarding Sentec, third party and/or open-source
softwareusedintheLuMon Systemisavailableatthefollowingwebpage:https://www.Sentec.com/licenses
© 2020 Sentec AG:Thecontentsofthis maynotbereproducedinanyformorcommunicatedto
anythirdpartywithoutthepriorwrittenconsentofSentecAG.
While every effort is made to ensure the correctness of the information provided in this , Sentec
AGassumesnoresponsibilityforerrorsoromissions.This issubjecttochangewithoutnotice.
Any serious incident that has occurred in relation to the device shall be reported to the manufacturer and the
competentauthorityoftheMemberState inwhichtheuserisestablished.
LuMon Monitor
SensorBeltConnector
LuMon Connector
SensorBelt
LuMon Belt
ContactAgent
NeoContactAgent
LuMon ContactSpray
Manufacturer:
SentecAG|Kantonsstrasse14|7302Landquart|Switzerland|www.Sentec.com
EU representative:
SentecGmbH|Carl-Hopp-Straße19A|18069Rostock|Germany

Introduction
fortheLuMon System
3of93
CONTENT
1INTRODUCTION.................................................................................................................................................................................5
1.1 LUMON SYSTEM .....................................................................................................................................................................5
1.2 ABOUT THIS USER SGUIDE.....................................................................................................................................................6
1.3 SYMBOLS,TERMINOLOGY AND ABBREVIATIONS ...............................................................................................................6
2SAFETY INFORMATION..................................................................................................................................................................7
2.1 SAFETY SYMBOLS AND MESSAGES ........................................................................................................................................7
2.2 WARNINGS AND CAUTIONS ....................................................................................................................................................7
2.3 EMC-RELATED SAFETY INFORMATION ..............................................................................................................................11
2.4 GLOSSARY OF SYMBOLS ........................................................................................................................................................12
3INDICATIONS AND CONTRAINDICATIONS.......................................................................................................................14
3.1 INTENDED USER .......................................................................................................................................................................14
3.2 INTENDED USE:GENERAL .....................................................................................................................................................14
3.3 INTENDED USE:INDICATIONS FOR USE .............................................................................................................................14
3.4 CONTRAINDICATIONS ............................................................................................................................................................14
4PRINCIPLE OF OPERATION AND LIMITATIONS..............................................................................................................15
4.1 GENERAL PRINCIPLE OF OPERATION .................................................................................................................................15
4.2 PRINCIPLE OF OPERATIONS WHEN USED FOR LUNG FUNCTION MONITORING.......................................................15
4.3 PARTICULAR FEATURES OF SENTEC EIT ...........................................................................................................................16
4.4 LIMITATIONS OF EIT/SENTEC EIT.......................................................................................................................................16
5SYSTEM OVERVIEW.......................................................................................................................................................................18
5.1 LUMON MONITOR...............................................................................................................................................................18
5.2 BELT CONNECTORS ................................................................................................................................................................19
5.3 BELTS.........................................................................................................................................................................................20
5.4 CONTACT AGENT/SPRAY.......................................................................................................................................................21
5.5 MEASURING TAPES ................................................................................................................................................................22
6LUMON MONITOR GUI.........................................................................................................................................................24
6.1 GUIOVERVIEW AND NAVIGATION.....................................................................................................................................24
6.2 SCOUTVIEW..............................................................................................................................................................................27
6.3 LUFUVIEW...............................................................................................................................................................................29
6.4 VENTVIEW................................................................................................................................................................................33
6.5 COMMON ASPECTS OF EITIMAGES AND TRENDS..........................................................................................................34
6.6 VISUAL INDICATORS...............................................................................................................................................................36
6.7 OPERATOR-ADJUSTABLE PARAMETERS ...........................................................................................................................40
7INSTALLATION AND PREOPERATIONAL CHECK..........................................................................................................42
7.1 USING THE EQUIPOTENTIALITY TERMINAL CONNECTOR..............................................................................................42
7.2 POWER SUPPLY.......................................................................................................................................................................42
7.3 SWITCHING THE LUMON MONITOR ON AND CHECKING ITS SYSTEM SETTINGS ..................................................43
7.4 PREOPERATIONAL CHECK ...................................................................................................................................................43
7.5 MINIMUM REQUIREMENTS ..................................................................................................................................................44
8BELT APPLICATION AND INITIATING MONITORING ..................................................................................................45
8.1 SENSORBELT APPLICATION AND INITIATING MONITORING FOR ADULTS AND CHILDREN ...................................45

Introduction
4of93
fortheLuMon System
8.2 LUMON BELT ADULT APPLICATION AND INITIATING MONITORING FOR ADULTS AND CHILDREN..................47
8.3 LUMON BELT NEO APPLICATION AND INITIATING MONITORING FOR NEONATES AND INFANTS ...................49
8.4 CHECKING THE PROPER SETUP............................................................................................................................................51
8.5 CHECKING THE ADEQUACY OF THE SELECTED ANALYSIS MODE ..............................................................................55
9WHILE MONITORING THE PATIENT..................................................................................................................................... 58
9.1 SELECTION OF PATIENT-SPECIFIC,CT-DERIVED THORAX AND LUNG MODELS .....................................................58
9.2 ACCOUNTING FOR THE PATIENT S POSITION...................................................................................................................59
9.3 QUALITY OF CALCULATED EITDATA................................................................................................................................ 60
9.4 GLOBAL DYNAMIC IMAGES AND PLETHYSMOGRAM......................................................................................................61
9.5 BREATH DETECTION...............................................................................................................................................................63
9.6 RESPIRATORY RATE ...............................................................................................................................................................64
9.7 ANALYSIS MODES...................................................................................................................................................................64
9.8 TRENDS OF EELI, EILIAND AERATION.............................................................................................................................66
9.9 STRETCH IMAGE RELATIVE TIDAL STRETCH..................................................................................................................67
9.10 CENTER OF VENTILATION,SILENT SPACES AND FUNCTIONAL LUNG SPACES ......................................................69
9.11 OPERATOR EVENTS AND SCREENSHOTS ..........................................................................................................................70
9.12 RECORDING OR EXPORTING DATA......................................................................................................................................71
10 PAUSING OR ENDING MONITORING...................................................................................................................................73
10.1 PAUSING MONITORING WITHOUT BELT REMOVAL.........................................................................................................73
10.2 PAUSING MONITORING WITH BELT REMOVAL AND/OR REPLACEMENT ...................................................................73
10.3 ENDING MONITORING...........................................................................................................................................................73
11 MAINTENANCE................................................................................................................................................................................74
11.1 ROUTINE CHECKS,PREVENTIVE MAINTENANCE AND SAFETY CHECKS..................................................................74
11.2 CLEANING AND DISINFECTION ...........................................................................................................................................74
11.3REPLACING THE FUSES OF THE LUMON MONITOR....................................................................................................76
11.4 DISPOSAL OF PARTS OF OR REMOVED FROM THE LUMON SYSTEM.......................................................................76
11.5 INSTRUCTIONS FOR REPACKING AND SHIPPING .............................................................................................................76
12 TROUBLESHOOTING....................................................................................................................................................................77
13 TECHNICAL SPECIFICATIONS.................................................................................................................................................78
13.1 SYSTEM PERFORMANCE .......................................................................................................................................................78
13.2 SYSTEM CHARACTERISTICS,COMPLIANCE AND COMPATIBILITIES ............................................................................78
13.3 LUMON MONITOR .............................................................................................................................................................80
13.4 BELT CONNECTORS ................................................................................................................................................................81
13.5 BELTS.........................................................................................................................................................................................82
13.6 CONTACT AGENT....................................................................................................................................................................82
13.7 DEVICE CLASSIFICATION ......................................................................................................................................................82
13.8 EMC DECLARATION ..............................................................................................................................................................83
14 ANNEX................................................................................................................................................................................................. 85
14.1 LMS RELATED ACCESSORIES,KEY SPARE PARTS AND DOCUMENTS.......................................................................85
14.2 TERMINOLOGY........................................................................................................................................................................85
14.3 ABBREVIATIONS USED IN THIS USER S GUIDE ................................................................................................................ 90
14.4 REFERENCES ...........................................................................................................................................................................92
fortheLuMon System
5of93
1INTRODUCTION
1.1 LuMon System
The LuMon System (LMS) is a compact and lightweight Electrical Impedance Tomography (EIT) system
providing noninvasive monitoring of variations of regional air content/volume within a cross-section of the
. It displays the results as real-time
EIT images
,
waveforms
,
parameters
and
indices
.
NOTE
The LuMon System is not intended for diagnosis, it is intended only as an adjunct in patient assessment. It
mustbeusedinconjunctionwithclinicalsignsandsymptoms.
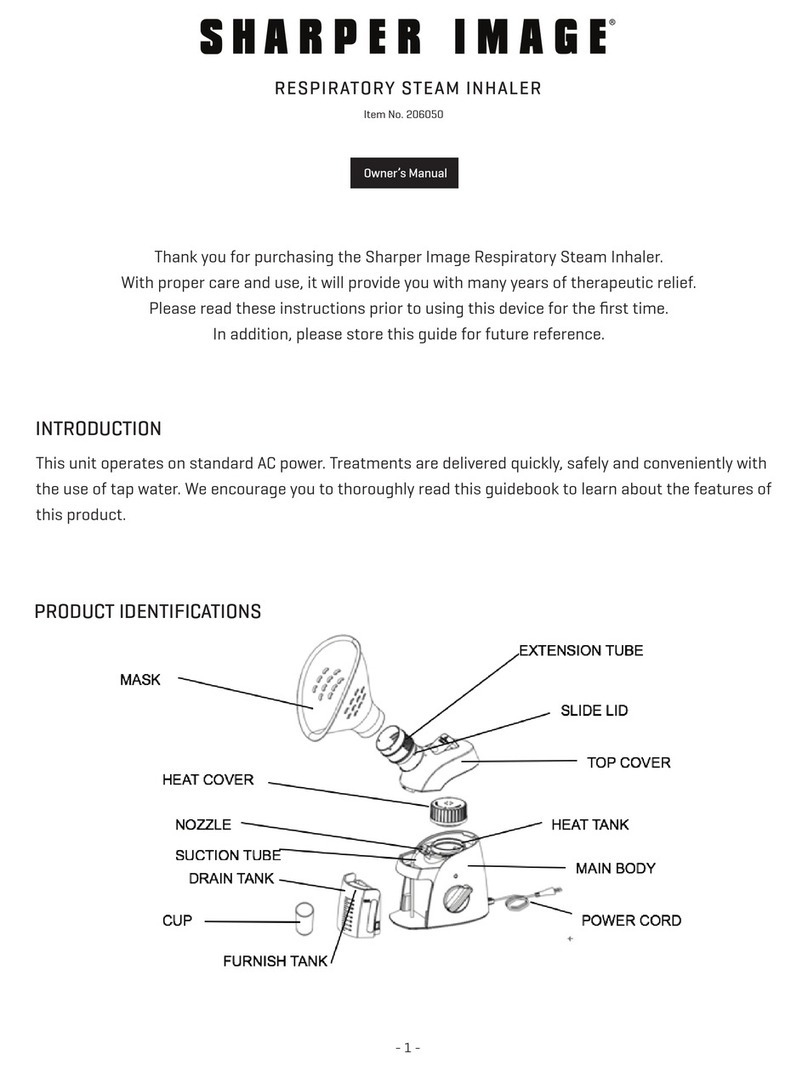
The LuMon System comprises LuMon Monitors (5.1), belt connectors (5.2) to link Sentec belts
(5.3) being available in various sizes to the LuMon Monitor, as well as Sentec contact agents/sprays (5.4)
serving as a medium for impedance coupling between a belt Measuring tapes (5.5)
permit the user to determine the recommended belt size, i.e. the size of the belt best fitting the respective
patient.
TheLuMon Systemisavailableintwoconfigurations
•for adults and children illustrated in Table 1-1 with a family of belts supporting an underbust girth
rangeofapproximately78to130cm(abbreviatedasLMS-A).
•for neonates and infants illustrated in Table 1-2 with a family of belts supporting an underbust girth
rangeofapproximately17 to52cm(abbreviatedasLMS-N).
Table1-1:LuMon System Adults/Childrenconfiguration( ) SensorBelt&ContactAgent
LuMon Monitor - Adult
SensorBeltConnector
SensorBelt
ContactAgent
Table1-2:LuMon System Adults/Childrenconfiguration( ) LuMon Belt&LuMon ContactSpray
LuMon Monitor - Adult
LuMon Connector
LuMon Belt Adult
LuMon Contact Spray
Table1-3:LuMon System Neonates/Infantsconfiguration( )
LuMon Monitor - Neo
LuMon Connector
LuMon Belt Neo
NeoContactAgent
Introduction
6of 93
fortheLuMon System
1.2
T rate and maintain the LuMon System. Before
attempting to operate the LuMon System, read this and pay special attention to the safety
information(2).Strictlyobserveallwarningsandcautions.
LuMon System indications and contraindications are provided in section 3. The principle of operation and
limitations of EIT in general and Sentec EIT in particular are elucidated in 4. A brief description of the
components of the LuMon System is provided in 5. Section 6describes the Graphical User Interface (GUI) of
theLuMon Monitor.HowtoinstalltheLuMon Systemisexplainedin7.
Section 8 explains how to apply the belts and to initiate monitoring, a description of the information displayed
whilemonitoringisprovidedin9,andhowtopauseorendmonitoringis outlinedin10.
Maintenance and troubleshooting related information are provided in 11 and 12, respectively. The technical
specificationsfortheLuMon System,finally,areprovidedinsection13.
NOTE
Statements in LuMon Monitors with the software versions indicated
on the cover page, an be any number. The software versions of the LuMon Monitor GUI and TIC
(0) - are displayed in the area of ScoutView (Figure 6-8). If your LuMon Monitor has other
so
GuidefortheLuMon System(seewww.Sentec.com/education/eit/plpm-eit).
1.3 Symbols, terminologyand abbreviations
Safetysymbolsandmessagesaredefinedinsub-section2.1.
A glossary of symbols used on the products of the LuMon System (1.1), on their packaging and in the
associateddocumentationisprovidedinsub-section2.4.
Ali -section14.2.
•Table 14-1 defines generalized product terms for those products of the LuMon System that have
different variants. Unless the differentiation between the product variants is of importance for a
specificcontext,thegeneralizedproducttermswillbeused.
•Table14-2definesSentecEITrelatedterms.
Notice that those terms being italicized and/or capitalized in Table 14-2 are
italicized
and/or Capitalized
throug
Also notice that the symbol ( ) identifies information and instructions being applicable only to the
Adults/Children configuration of the LuMon System whereas the symbol ( ) identifies information and
instructionsbeingapplicabletoitsNeonates/Infantsconfigurationonly.
din0.

fortheLuMon System
7of93
2SAFETYINFORMATION
2.1 Safetysymbols and messages
Safetysymbolsandmessagesareshownanddefinedasfollows:
WARNING
Warnings alert users to potential serious outcomes (death, injury, or adverse events) to the patient, user, or
environment.
CAUTION
Cautions indicate a potentially hazardous situation, which, if not avoided, could result in minor or moderate
injury.
NOTE
Notesprovideadditionalguidelinesorinformation.
A comprehensive lits of warnings and cautions are provided in 2.2. Some are repeated in other sections of the
Us oreinforceaspecificsafetytopic.Notesareprovidedinsectionswhereapplicable.
2.2 Warnings andcautions
WARNING
The LuMon System is to be operated by qualified health care personnel. Personnel operating the LuMon
System should have read and understood this manual, accessory directions for use, all precautionary
information, and specifications before use. Improper use of the LuMon System may result in injury, inaccurate
measurementsand/ordamagetothe device.
WARNING
VisuallycheckNeoContactAgentbeforeuseforindicationofmicrobialgrowth.
WARNING
Use only equipment, accessories, disposables, or parts supplied or recommended by Sentec AG. Use of other
partsmayresultin injury,inaccuratemeasurementsand/ordamagetothedevice.
WARNING
OnlyuseSentec contactagents/spraysto wetthebelt eanyother
agents or liquids such as ECG or ultrasonic gel. Doing so may adversely affect the belt measurement and the
performanceoftheLuMon System.
WARNING
Measurements and displayed images of the LuMon System may be affected by patientmanipulation
ormovement.
WARNING
Donot useLuMon Monitors,beltconnectors orbeltsthat appeardamagedorhavea technicalfault.Doingso
mayresultininjury,inaccuratemeasurementsand/ordamagetothedevice.
WARNING
To reduce the possibility of patient entanglement or strangulation, properly route and fix cables. Do not leave
unappliedbeltsinreachofthepatient.
WARNING
Toensurepatientsafety,do not placetheLuMon Monitor,mountedorunmounted,inany positionthat might
cause it to fall ortip over on the patient. Ensure to properly fasten the LuMon Monitor, when mounting it on,
for example, a roll stand or a wall mount/railing. Furthermore, do not lift the LuMon Monitor by the belt
connectororthe ACpowercordbecausetheycoulddisconnectfromthe LuMon Monitor,causingit to fallon
thepatient.
WARNING
BeforecleaningtheLuMon Monitor,alwaysswitchitoffanddisconnect itfromACmainspower.

SafetyInformation
8of93
fortheLuMon System
WARNING
Apartfromusing cleaninganddisinfectionagentsasrecommendedherein,donotspray,pour,orspillanyliquid
on LuMon Monitors (particularly on the openings of its chassis), belt connectors, belts and other accessories.
NeitherimmerseanypartsoftheLuMon Systemnorallow waterorother liquidsto enterthe device.Failureto
do so increases the risk ofelectrical shock and/or may result in damage to the device. If a LuMon Monitor has
been wetted accidentally, it must be removed from AC mains power, wiped dry externally, allowed to dry
thoroughly,and inspectedbyqualifiedtechnician(e.g.abiomedicalengineer)beforefurtheruse.
WARNING
Do not expose the LuMon Monitor to heavy moisture and do not allow any fluids to enter the LuMon
Monitor.Plugsandconnectorshavetobekeptmeticulouslycleananddryatalltimes.Failuretodosoincreases
theriskofelectricalshockand/ormayresultindamagetothedevice.
CAUTION
Disposeofthe batteryinaccordancewithlocalrequirementsandregulationsforLithiumIonbatteries.
WARNING
Explosionandflammabilityhazards.Donot usethe LuMon Monitorin thepresenceof flammableorexplosive
anesthetics/gases or other flammable or explosive substances. The LuMon Monitor is not rated for use in an
oxygenrichenvironment.
WARNING
The ContactAgent and LuMon Contact Spray are extremely flammable aerosols. Keep away from
heat/sparks/open flames/hot surfaces. No smoking. Do not spray on an open flame or other ignition source.
Pressurized container: Do not pierce, crush or burn, even after use. Protect from sunlight. Do not expose
totemperaturesexceeding50°C/122°F.Failuretodosoincreasestheriskoffireand/orexplosion.
Caution
WARNING
Before using the LuMon System verify that it does not interfere with bioimpedance measurement devices
such as impedance respiration monitoring or with ECG, EMG, EOG or EEG devices being connected to the
samepatient.Interferencewithsuchdevicesmayadverselyaffectthemonitoringofthepatient.
WARNING
Do not use the LuMon System on patients with internal or external pacemakers or other active implants such
asdefibrillators.TheLuMon Systemmayaffecttheoperationofsuchdevices.
WARNING
The LuMon System is NOT rated for use with a defibrillator. Therefore, remove the belt from the patient
before defibrillating a patient. Failure to do so may reduce the defibrillation effectiveness or cause device
damage.
WARNING
This device has been tested and found to comply with the requirements for medical devices according to the
IEC 60601-1-2. These requirements are designed to provide reasonable protection against harmful
interferenceina typicalmedicalinstallation.Wheninterpretingmonitoreddatabeawarethatinterferencesthat
either affect the LuMon System and/or other devices may nevertheless occur and, hence, may adversely
affectthemonitoringofthepatient.Incase youwitness orsuspectinterferences,contacta qualifiedtechnician,
yourlocalSentecEITrepresentativeorSentecAG.
WARNING
Portable RF communications equipment (including peripherals such as antenna cables and external antennas)
should be used no closer than 30 cm (12 inches) to any part of the LuMon System. Otherwise, degradation of
theperformanceoftheLuMon Systemcouldresult.
WARNING
The LuMon Monitor should not be used adjacent to or stacked with other equipment as these can cause
electromagnetic interference and thereby result in incorrect measurements. If adjacent or stacked use is
necessary, the LuMon Monitor should be observed to verify normal operation in the configuration it is to be
used.
WARNING
High-frequency surgical equipment may influence the operation of the LuMon System and may not be
operatedincombinationwiththeLuMon System.
fortheLuMon System
9of 93
WARNING
MR UNSAFE. Do not use the LuMon System with magnetic resonance (MR) equipment. Induced current in
the belt and belt connector could potentially cause patient burns, and the MR image quality could be affected
bytheLuMon SystemandtheLuMon SystembytheMRequipment.
WARNING
During normal operation (except intra-hospital transport), it is recommended that the monitor is always
connectedto ACmainspower.
WARNING
If the monitor is operated on an AC mains power source with a depleted battery and the AC mains power is
subsequentlydisconnectedorlost,themonitormayshutdownimmediately.
WARNING
Do not connect the LuMon Monitor to an electrical outlet controlled by a wall switch, because the LuMon
Monitor may be unintentionally disconnected from AC mains power and, once the battery is depleted,
accidentallyswitchoff.
WARNING
To avoid risk of electrical shock, this equipment must be connected to AC mains power with protective earth.
Ensure that power and protective ground lines are connected correctly. As a precaution connect this
equipment directly to fixed wall sockets within hospitals or hospital type facilities only. Neither connect it to
portablesocketsnoruseextensioncordsorconnectittopubliclyaccessibleACmainspower.
ForUS, respectivelyJapan: Grounding reliability canonly beachievedwhenthe LuMon Monitoris connected
toanequivalentreceptaclemarkedHG(HospitalGrade),respectivelyHGJ(HospitalGradeJapan).
WARNING
Accessory equipment (e.g. a PC or an externally powered USB memory device) connected to the LuMon
Monitor certifiedaccordingtotheapplicableIECstandards(e.g.IEC60601-1,UL60601-1,
CSA C22.2 No. 601-1-M90, or IEC 60950). Furthermore, all resultingconfigurations must comply with the IEC
standard 60601-1 systems requirements. Anyone who connects accessory equipment to the LuMon Monitor
configures a medical system andis, therefore, responsible for ensuring that the resulting system complies with
the IEC standard 60601-1 systems requirements and the electromagnetic compatibilitystandard IEC 60601-1-
2. Connection of accessory equipment to the LuMon Monitor`s data ports is to be performed by qualified
personnel.
WARNING
SensorBelts and LuMon Belts, are for single patient use only do not attempt to reuse, clean, disinfect, or
sterilize.Usingabelt onmorethanonepatientincreasestherisksofinfectionandcross-contamination.Usinga
belt whose
belt time
has expired may compromise its biological integrity and functionality as well as the overall
system performance. Dispose of the belt when the
belt time
has elapsed or after ending monitoring for a
patient.
WARNING
Do not apply SensorBelts, LuMon Belts, ContactAgent, LuMon Contact Spray or NeoContactAgent on
open,uncoveredwounds.Doingsoincreasestherisksofinfectionandtissueirritation.
CAUTION
Biological evaluation has been conducted in compliance with ISO 10993-1 on the belts and the contact
agents/sprays for their useontheintactskin ofan individual patientfor up to30 cumulativedays. Nevertheless,
on rare occasions erythema (skin redness) has been observed in neonates and infants on the skin area where
the belt has been applied. Should skin redness occur, it will generally resolve within a few hours after belt
.
WARNING
WipeoffContactAgentresiduefromthepatientafterbeltremoval.
WARNING
Clean and disinfect the reusable parts of the LuMon System
monitoringforapatientand beforeusewithanewpatient,andregularlyaccordingto institutionalpolicy during
use with a single patient. Before each use, prepare the LuMon System as described herein. Failure to do so
increasestheriskofcrosscontaminationandpatientinfection.
WARNING
Allparts of orremovedfromtheLuMon Systemmustbeconsideredpotentiallycontaminatedandasourceof
infectionrisk.Disposeofallpartsremovedfromthedeviceaccordingtolocalregulationsformedicalwaste.

SafetyInformation
10of93
fortheLuMon System
WARNING
The SensorBelt generate a small amount of heat and their surfaces can
reachseveral°Cabove ambienttemperature.Failuretoadheretothefollowingpointsincreases therisk oflocal
skinburn:
1) Do not place the MatchBox directly on the patient and place the ControlBox in a way to prevent any skin
contactwiththepatient.
2) Where possible do not cover the ControlBox or the MatchBox with linens or bed covers to avoid possible
heatingoftheskin.
3) To minimize the pressure on the chest of the patient in prone position right there where the MatchBox is
inserted in the SensorBelt
MatchBoxplace,forexample,twosmallcushionsoneithersideofthedockingstation.
WARNING
The LuMon ates a small amount of heat and its surface can reach several °C
aboveambienttemperature.Failuretoadheretothefollowingpointsincreasesthe riskoflocalskinburn:
1) PlacetheControlBoxinawaytopreventanyskincontactwiththepatient.
2) Where possible do not cover the ControlBox with linens or bed covers to avoid possible heating of the
skin.
CAUTION
Beawarethatabeltbeingappliedtoa patientmayimpairthequalityofx-rayimages(e.g.ofchestx-rays).
CAUTION
To avoid misinterpretation of results, be aware that in some situations Lung Impedance changes displayed by
the
Plethysmogram
may be less related to breathing than to other sources such as cardiac activity, therefore
one should not rely solely upon the visual representation of the
Plethysmogram
, i.e. the
Lung Impedance
waveform
,toderiveinformationonbreathing.
WARNING
Dataqualitymaybeimpaired,measurementsincorrectandthusresultsmaybemisinterpretedif
1) thesizeoftheconnectedbeltdoesnotmatchtherecommendedsize
2) thebeltisnotappliedandpositionedasrecommended
3) rotationandinclinationdisplayedbytheLuMon Monitor position.
4) patient data, half/full underbust girth or belt displacement are not determined and entered correctly.
Notice that these settings are reset to their defaults when starting up the monitor. You therefore must re-
enterthesesettingsaftereachrestartofthemonitor
5) aninadequateAnalysisModeisselected.
CAUTION
In
TB-I mode
the minima and maxima and, hence, the maximal
Lung Impedance
changes detected within
Analysis Intervals
are, irrespective of the magnitude or rate of the
Lung Impedance
changes, assumed to be
related to breathing, more specifically to end-expiratory and end-inspiratory time points. In order to avoid
misinterpretation of data, be awarethat
breathing-relatedEIT images and indices
generatedin
TB-Imode
may
consequentlynotalwaysbephysiologicallymeaningful.
CAUTION
To avoid misinterpretation of results be aware that the
Dependent Silent Spaces
, the
Non-Dependent Silent
Spaces
as well as the vertical and horizontal components of the
Center of Ventilation
(CoV(v) and CoV(h)) are
less meaningful, if the gravity vector is more or less perpendicular to the examined thorax cross-section being
definedbythe
beltplane
.Thisis,forexample,thecase,ifthepatientisstandingorsittingupright.
CAUTION
To avoid misinterpretation of results, it should be considered that the displayed
Thorax
and
LungContours
and
associatedthoraxandlungmodelsusedbythe LuMon Systemtoevaluatethemeasured
EITdata
maydeviate
significantly from reality in case of patients with, for example, anatomic anomalies (e.g. after lung resection) or
pathologies.Donotutilizethedisplayed
Thorax
and
LungContours
fordiagnosticpurposesorevaluation.
CAUTION
Use of other cleaning and disinfection agents than recommended may cause damage and/or deterioration of
anddevicefailuremayresult.
CAUTION
Applying excessive materials and
devicefailuremayresult.
fortheLuMon System
11of93
CAUTION
Do not touch, press or rub the surfaces of the LuMon Monitor or belt connector with abrasive cleaning
compounds, instruments, brushes, rough surface materials, or bring them into contact with any that could
scratchthesurfacesoftheLuMon Monitororbeltconnector.
CAUTION
Donotusepetroleum-basedoracetonesolutions,orotherharshsolvents,tocleantheLuMon Monitororbelt
connector.Thesesubstancescanattackthedevicematerialsanddevicefailuremayresult.
CAUTION
TheLuMon Systemanditsaccessoriesareprovidednon-sterile.Donotsterilizeanypartsoftheequipmentby
irradiation,steamorethyleneoxide.Donotautoclaveorpressuresterilize.
CAUTION
DonotuseSensorBeltsorLuMon Beltsifpackagingisdamaged.
WARNING
There are no user serviceable parts inside the LuMon Monitor. The cover of the LuMon Monitor should only
be removed by
Sentec Authorized LuMon Technicians
. Service as well as the complete safety and
functionality test should be made by qualified technicians. Failure to do so could lead to injury, inaccurate
measurementsand/ordamagetothedevice.
WARNING
Do not modify the LuMon System without express approval from Sentec. Modifications to the system by
persons without the appropriate training or using unapproved parts could lead to injury, inaccurate
measurementsand/ordamagetothe device.
WARNING
RemoveLuMon Beltifsoiled.
WARNING
TheLuMon Monitorisnotanapneamonitor.
2.3 EMC-relatedsafetyinformation
TheLuMon Systemisintendedforuseintheelectromagneticenvironmentspecifiedin 13.8.
2.3.1 Electromagneticemissions
This equipment generates, uses, and can radiate radio frequency energy and, if not installed and used
according to the User's Guide, may cause harmful interference with radio communications. Operation of this
equipment ina residential area is likely tocause harmful interference,in which case theusers willbe required to
correcttheinterferenceattheirownexpense.
The LuMon Monitor has been tested and found to comply with the limitsfor a Class A digital device, pursuant
to both Part 15 of the FCC rules and the radio interference regulations of the Canadian Department of
Communications.
NOTE
The emissions characteristics of this equipment make it suitable for use in industrial areas and hospitals (CISPR
11 Class A). If it is used in a residential environment (for which CISPR 11 class B is normally required) this
equipment might not offer adequate protection to radio-frequency communication services. The user might
needtotakemitigationmeasures,suchas relocatingorreorientingtheequipment.
2.3.2 Electrostatic discharge precautions
Always use proper electrostatic discharge (ESD) procedures, protection, and products when handling and
before operating the device. Failure to use ESD procedures may damage electrostatically sensitive
components in the device.Such damageto components is not covered by Sentec warranties. ESD can amount
to a significant voltage that can damage PCBs (printed circuit board) or other system components. ESD
damage is cumulative and may not be apparent at first, causing only degraded performance rather than a clear
actual device failure. ESD is more likely to occur under low humidity conditions, or through contact with
carpeting,linens,orclothing.

SafetyInformation
12of 93
fortheLuMon System
2.3.3 Electromagneticsusceptibility
TheLuMon MonitorcomplieswithIEC60601-1-2 EMC(ElectroMagneticCompatibility)CollateralStandard.
Certaintransmittingdevices(forexample.cellularphones,walkie-talkies,cordlessphones,pagingtransmitters),
however,emitradiofrequenciesthatcouldinfluenceordisturbtheoperationoftheLuMon Monitor.
2.4 Glossaryofsymbols
The table below summarizes symbols used on the LuMon System (including all its related parts), on the
packaging and in the associated documentation. These symbols indicate information essential for proper use;
theorderoftheirappearanceisnotprioritized.
Followinstructionsfor use
Consultinstruction foruse
Manufacturer
Date ofmanufacture
EU authorizedrepresentative
uropéenne t
thedeviceisinconformancewith the
applicableEuropean Regulationsand
Directivesasstated inthedeclaration of
conformity.
Symbolforconformitytotheaerosol
directive75/324/EEC
MedicalDevice
Referencenumber
Serial number
BatchorLot number
Rx only
PrescriptionDevice
IPxy
Ingressprotectionclass xy
Fuse
Radiofrequency transmitter
AppliedparttypeBF(accordingtoIEC
60601-1)
Off(power)
On(power)
USBconnection
ACPower/Battery indicator
IOIO
Serialport
Equipotentialityterminal
Ethernetconnection
Single-use
Singlepatientmultipleuse
Indicatorof beltsize (underbust
circumferenceincm)
Usebydate
Nominalquantityinformationabout
content
Caution
Extremelyflammableaerosol
Keepdry
Keepawayfrom sunlight
Humiditylimit
Pressurelimit
Temperaturelimit
Upperlimitoftemperature
Do notuse if packageis damaged
Disposeofaccording to Council
Directive2012/19/EU
Recommendeduser action(s)
Information/instructionsapplicable to
theAdults/Children configurationof
LuMon System

SafetyInformation
fortheLuMon System
13of93
Information/instructionsapplicable to
theNeonates/Infants configurationof
LuMon System
IngredientsinINCInomenclature
Storeinanuprightposition
Transport
Storage

14of93
sGuidefortheLuMon System
3INDICATIONSANDCONTRAINDICATIONS
3.1 Intendeduser
It is intended that the LuMon System (1.1) will be used only by licensed health care practitioners who
understanditsfundamentalfunctioningand principleofoperation(4).
NOTE
Personnel operating the LuMon System should have read and understood this manual, accessory directions
foruse,allprecautionaryinformation,andspecificationsbeforeuse.
3.2 Intendeduse:General
The LuMon System (1.1) is intended for use in patient requiring assessment or monitoring of respiration and
(regional) lung function. This includes patients breathing spontaneously or requiring supplemental oxygen,
breathing support or mechanical ventilation. It is also intended to monitor ventilation distribution in patients
lying, for example, in supine, prone, and lateral positions, where regional lung volume distribution is of clinical
interest.
The LuMon System is intended for use under direct supervision of licensed healthcare practitioners in
professionalhealthcarefacilitiessuchashospitals,hospitals-typefacilities,orintra-hospitaltransport.
3.3 Intendeduse:Indications forUse
The LuMon System (1.1) consisting of the LuMon Monitor and specified accessories is a noninvasive,
non-radiologicalmonitoringdevicethatprovidesanassessmentofregionalimpedancevariationwithina cross-
section of a patient's thorax. Graphical and numericalinformationis presented to the clinical user to support an
assessme s lungs. The
LuMon System provides no alarms and its measurements are only to be used as an adjunct to other clinical
information.TheLuMon Systemis notintendedtobeutilizedasa primaryvitalsignsmonitor.
The SensorBeltConnectorand theThe LuMon Connector are reusable adapters intended to link a SensorBelt
oraLuMon BelttotheLuMon Monitor.
The SensorBelts and the LuMon Belts are non-sterile, single-patient use accessories, and are intended to be
s thorax on intact skin. Sequential application of SensorBelts and
LuMon Beltsonasinglepatientcanberepeatedforupto30cumulativedays.
The SensorBelts are indicated for use in adults and pediatric patients with selection of an appropriate belt size
basedonthethoraxcircumference(underbustgirth).
The LuMon Belts are indicated for use in adults through neonatal patients with selection of an appropriate
beltsizebasedonthethoraxcircumference(underbustgirth).
The
ContactAgent
,the
LuMon ContactSpray
and the
NeoContactAgent
arecontactmediaintendedfor use
with the
SensorBelts
and the
LuMon Belts
for conductive coupling between the belts and the intact skin of
patients.The
ContactAgent
and the
NeoContactAgent
are non-sterile and are intended for use on the intact
skin of an individual patient for up to 30 cumulative days. The LuMon Contact Spray is non-sterile and
intendedforuse on theintactskinofadultsandchildrenforupto30cumulativedays.
The LuMon System is intended for use under the direct supervision of a licensed healthcare practitioner in
professionalhealthcarefacilitiesprovidingpatientcare.
NOTE
TheLuMon SystemisnotcurrentlyFDAclearedforuseintheUSA.
3.4 Contraindications
Using the LuMon System (1.1) is contraindicated if any of the following conditions are present or are thought
tobepresent:
•Thepatienthasactiveimplants(pacemakerordefibrillator).
•Thepatienthasanexternalpacemakerordefibrillator.
•Thepatient hasopen/uncoveredwounds inthe areawherethe beltisapplied, asforexample anopen
thoraxduring/afterheartsurgery.

Principleofoperationandlimitations
fortheLuMon System
15of93
4PRINCIPLEOFOPERATIONANDLIMITATIONS
4.1 Generalprinciple of operation
Sentec EIT is based on the principles of Electrical Impedance Tomography (EIT), where weak alternating
currents are applied and travel along the paths of least resistance through an object, and the electrical
poten nuously measured by an array of electrodes placed on the
object. Typically, electrode arrangements are designed to sequentially pick up signals coming from different
directions. In this way, the electr reflecting regional
impedancewithintheobjectandvariations thereofcanbecreatedwithframeratesoftypically50Hz.
Figure 4-1 illustrates this concept for a belt with embedded electrodes fastened around the chest of a subject.
Very weak, harmless alternating current is applied to a pair of electrodes (in red). The current applications are
successively shifting around the chest. For each current applied to a pair of electrodes, the voltages are
measuredby32pairsofelectrodes(inblue).
Figure4-1:SchematicdrawingoftheelectrodelocationsforanEITbeltfastenedaroundthechestofasubject.
4.2 Principleofoperationswhen usedforlungfunctionmonitoring
When a belt with embedded electrodes is fastened around the chest (Figure 4-1) it is possible to continuously
monitor and visualize regional impedance variations within a cross-
impedance variations are primarily caused by lung function i.e. air flowing in, distributing within, and flowing
out of the lungs and, to a lesser extent, perfusion and cardiac activity, it is possible to continuously monitor
variations of regional air content/volume ngs noninvasively, without radiation and at the
bedside. Various images (e.g. images relatedto distribution of tidal volume),
waveforms
as well as a wide variety
of
indices
and
parameters
canbederivedandtrended,includingbutnotlimitedto:
•
Plethysmogram
(9.4) representing relative variations of
Lung Impedance
, related to relative lung
volume/aircontentvariationswithbreathing.
•Impedance
RespiratoryRate
(RRi)(9.6).
•
End-Expiratory Lung Impedance
(EELI) (9.8) related to end-expiratory lung volume, i.e. if exhaling
against ambient pressure, to the Functional Residual Capacity (FRC), when breathing normally, or to
theResidualVolume(RV),inaforcedexpiration.
•
End-InspiratoryLungImpedance
(EILI)(9.8)relatedtoend-inspiratorylungvolume.
•
Tidal Variation
(TVi) (9.8), the difference between EILI and EELI, related to the volume inhaled in one
breath, i.e. to the Tidal Volume (TV), when breathing normally, or to the Inspiratory Capacity (IC), in a
forcedinspiration.
•
Aeration
(9.8) defined as the mean
Lung Impedance
(MLI) over a preset
Analysis Interval
of a fixed
durationof15seconds,relatedtomeanlungvolume.
•
Stretch Image
(9.9), displaying the regional distribution of
Relative Tidal Stretch
(RTS) and
representingtheregionaldistributionofTidalVolumes(TV)withinthelungs.
•
Center of Ventilation
(CoV) (9.10) characterizing the ventilation distribution and represented, for
example, in vertical and horizontal direction, with the CoV vertical component (CoV(v)) defining the
positionofthe
HorizonofVentilation
(HoV).
•
Silent Spaces
(9.10), reflecting the distribution and percentage of lung areas with little or no
impedance change during breathing. As such they are meant to represent the percentage of lung
regions receiving little or no ventilation and are thus hypoventilated. Considering the influence of
gravity on lung tissue and fluids within the lungs,
Silent Spaces
being localized above or below the
HoVarefurther definedas
DependentSilentSpaces
(DSS)and
Non-DependentSilentSpaces
(NSS),
respectively.
Silent Spaces
may be helpful to identify conditions such as displacement of the

Principleofoperationandlimitations
16of 93
fortheLuMon System
endotracheal tube, pneumothorax, and pleural effusion as well as conditions influenced by gravity
such as collapsed, fluid filled or distended lung areas, with DSS reflecting the first two conditions and
NSSthelatter.
•
Functional Lung Spaces
(FLS) (9.10) reflecting the distribution and percentage of lung areas with
non-negligible impedance change during breathing. As such they are meant to represent the
percentage of ventilated lung regions. They are related to what in literature is referred to as
Functional Lung Size [1] or size of the available lung volume, i.e. the percentage of the aerated
remaininglung.
The EIT methodology has been an object of study fordecades and there is plenty of literatureon the subject;a
summary description can be found for example in Costa et al.[2]. For more information on its clinical
applications, please also refer to reviews available in literature, e.g. Frerichs et al. [3], Putensen et al.[4], Lobo et
al.[5].
4.3 ParticularfeaturesofSentec EIT
Sentec EIT selects the thorax and lung models best adapted to the individual patient from a set of predefined,
CT-derivedthoraxandlung models (9.1).Thesemodelsareusedfor
EITimage
reconstructionandto determine
corresponding
Thorax
and
Lung ROIs
respectively
Thorax
and
Lung Contours
. In Adults/Children
configuration these models mainly depend on body mass index (BMI) for both genders. In Neonates/Infants
configuration, whereonly one CT-derived thoraxand lung model is used,the LuMon System accounts for the
displacement of the electrodes around the thorax from their assumed default positions. It is herewith
emphasized that, with the exception of the
Global Dynamic Image
(9.4), the LuMon System solely evaluates
impedancevalueswithinthe
LungROI
.
Sentec EIT also features a position sensor that continuously evaluates position (
rotation
and
inclination
) and permits the clinician to assess the influence of gravity on lung mechanics and ventilation
distributioninthelungs(9.2).
As fully functional electrodes are important for the generation of
EIT data
, the LuMon System continuously
evaluates the skin contact quality of all 32 electrodes integrated in the belt
and categorizes the electrodes into
those having adequate, poor, or insufficient contact, respectively impedance coupling to the skin, the latter
referred to as
failing electrodes
(9.3). The LuMon System uction algorithm
can compensate up to six
failing electrodes
. When the belt to skin contact quality changes significantly or a
change in the number of
failing electrodes
occurs, the LuMon System performs a calibration of its
measurement setup and monitoring is briefly interrupted. In case of too many
failing electrodes
monitoring is
not possible. Once data quality respectively signal quality (9.3) improves, for example, when there are fewer
failingelectrodes
,monitoringresumes.
4.4 LimitationsofEIT/SentecEIT
The following clinical situations or factors may affect
EIT raw data
and thereof derived
EIT images
,
waveforms
and
indices
andmaylimittheaccuracyofthereofderived
parameters
suchas the
RespiratoryRate
(RRi).
•Mal-positioned or not properly applied belt e.g. belt not positioned as recommended or not
enoughcontactagentappliedonbelt(8).
•Conditions hindering sufficient belt to skin contact quality such as bandages in the area around the
thoraxwherethestripedfabricofthe beltshastobeplaced.
•Conditions causing an abrupt or gradual deterioration of the belt to skin contact quality e.g.
(excessive)patientmovement/manipulationorgraduallooseningofthebelt fitaroundthechest.
•Conditions (transiently) causing impedance changes within the
EIT sensitivity region
not related to
breathing e.g. administration of fluids or movement of liquid and tissue (e.g. diaphragm) within the
EITsensitivityregion
.
•Use of
TB-I Analysis Mode
in very irregularly and/or very weakly breathing patients, in which the
maximal
Lung Impedance
change within
Analysis Intervals
may not be
breathing-related
and, hence,
breathing-related EIT images and indices
generated in
TB-I mode
may not always be physiologically
meaningful(8.5,9.7).
•Use of Sentec EIT in patients after lung resection or having thoracic malformations in this case, the
models for thorax and lung
may deviate significantly from reality, a fact that should be considered in
theinterpretationoftheresults.
•UseofSentecEITwhenitiscontraindicated(3.4).
•Interferences caused by other devices such as high-frequency (HF) surgical equipment or devices
emittingstrongelectromagneticfields.
•The subdivision of
Silent Spaces
in
Dependent Silent Spaces
and
Non-Dependent Silent Spaces
as
well as the vertical and horizontal components of the
Center of Ventilation
(CoV(v) and CoV(h)) are
less meaningful if the
inclination
is big enoughthat the gravity vector is more orless perpendicular to
theexamined thoraxcross-sectionbeingdefinedbythe
beltplane
.This is,forexample,thecaseifthe
patientisstandingorsittingupright.

Principleofoperationandlimitations
fortheLuMon System
17of93
NOTE
EITdoesnot measurethe behavioroftheentirelung but onlythe lung regions locatedwithin the
EITsensitivity
region
.
EIT images
and thereof derived
parameters
and
indices
, consequently, do not represent information
being related the entire lung, but only information being related to the lung region located within the
EIT
sensitivityregion.
Being centered around the
belt plane
the
EIT sensitivity region
is roughly lens-shaped: close to the body
surface the thickness of the
EIT sensitivity region
corresponds toat least the width ofthe belt (Table 13-13) and
increases towards the central region of the thorax to reach a thickness of roughly one half to two-thirds of the
thoraxwidth.

Systemoverview
18of93
fortheLuMon System
5SYSTEMOVERVIEW
This section provides a brief description of each component of the LuMon System (1.1), which is available ina
configurationforadultsandchildrenandinaconfigurationforneonatesandinfants.
The LuMon System comprises LuMon Monitors (5.1), belt connectors (5.2) to link Sentec belts
(5.3) being available in various sizes to the LuMon Monitor, as well as Sentec contact agents/sprays (5.4)
serving as a medium for impedance coupling between a belt Measuring tapes (5.5)
provided with the contact agents/sprays permit the user to measure the patient to determine the
recommendedbeltsize,i.e.thesizeofthebeltbestfittingtherespectivepatient.
5.1 LuMon Monitor
The LuMon Monitor (Figure 5-1, Figure 5-2) is a portable, bedside stand-alone EIT monitor available in two
configurations:
•() The LuMon Monitor Adult, i.e. Adults/Children configuration, supports
SensorBeltConnectors (5.2.1) and SensorBelts (5.3.1) and/or LuMon Connector (5.2.2) and LuMon
BeltAdult(5.3.2)
•( )The LuMon Monitor Neonates/Infants configuration,supports LuMon
Connectors(5.2.2)andLuMon Belts Neo(5.3.3).
NOTE
The monitor housing, connectors and graphical user interface arecommon to bothmonitor configurations and
aredescribedbelow.
Figure5-1:LuMon Monitor frontpanel(hereaLuMon Monitor Adult)
A. Touchscreenbased GraphicalUser Interface GUI(6).
B. SupportfootindicatingtheLuMon Monitor
LuMon ADULT identifies theLuMon Monitor Adult;
LuMon NEO identifiesthe LuMon Monitor Neo.
NOTE
The LuMon Monitor screen supports finger touchoperation only. The use of rigid orsharp instruments on the
touchscreencanpermanentlydamagethedisplay.
NOTE
The LuMon Monitor performs a calibration of its touch screen after it is switched on. To not disturb the
calibrationofthetouchscreen,donottouchthescreenthefirstfewsecondsafterstartup.

Systemoverview
fortheLuMon System
19of 93
Figure5-2:LuMon Monitor rearpanel
A. Ventilationslot
B. ON/OFFswitch
C. ACPower/Battery indicator
D. Typelabel
E. Fuseholders(2x)
F. ACpowerinlet
G. Integratedcarrying handle
H. Beltconnectorsocket
I. USBports(2x) onlyforuse withmemory devices
withoutexternal powersupply
J. Serialports(2x) factory useonly
K. Ethernetport factory use only
L. Equipotentialityterminalconnector
M. Ventilationslot
N. VESA75compatiblemounting holes (4x)
5.2 Beltconnectors
The belt connectors are designed to link belts to LuMon Monitors. Belt connectors control the injection of
very weak alternating currents into the nd the measurement of the voltages (electrical
potentials)resultingattheskinofthepati ax.
5.2.1 SensorBeltConnector
TheSensorBeltConnector(Figure5-3)linksaSensorBelt(5.3.1)to aLuMon Monitor Adult(5.1).Theposition
sensor embedded in its MatchBox permits the LuMon System to m position
(
rotation
and
inclination
) (9.2). If the SensorBeltConnector is properly connected to a LuMon Monitor in on-
state,thestatusindicatorLEDofitsMatchBoxcontinuouslylightsgreenifaSensorBeltisconnected.
Figure5-3:SensorBeltConnector
A. MatchBox(includesa positionsensor anda
statusindicator LED) connectsto SensorBelts
B. ControlBox (withintegratedelectronics and
statusindicator LED)
C. Monitor plug connects to the belt connector
socketonthe LuMon Monitor
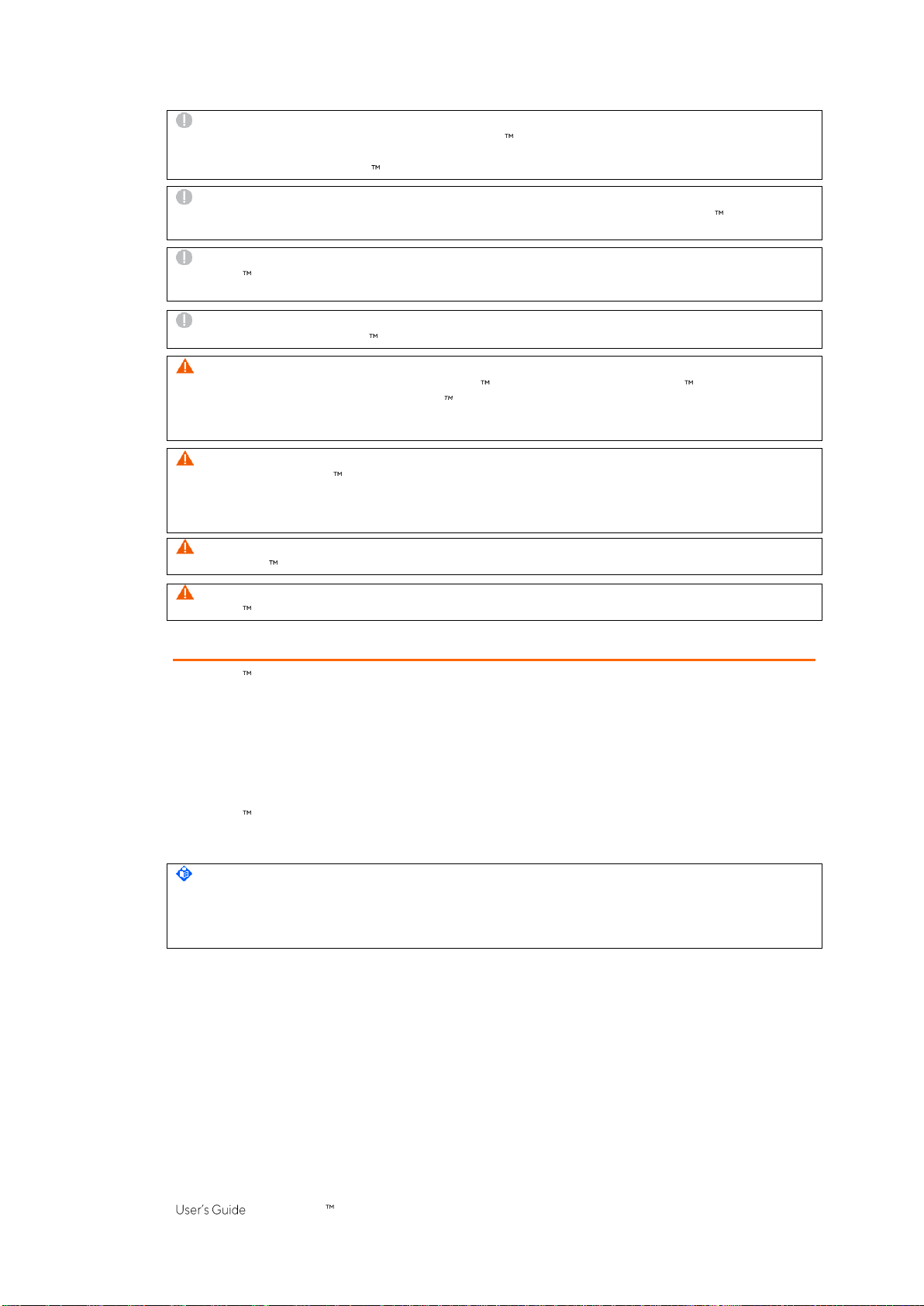
5.2.2 LuMon Connector
TheLuMon Connector(Figure5-4)linksaLuMon Belt(5.3.2/5.3.3)toa LuMon Monitor(5.1).
In contrast to the MatchBox of the SensorBeltConnector (5.2.1) the MatchBox of the LuMon Connector
(5.2.2)containsneitherapositionsensornorastatusindicatorLED.
Systemoverview
20of93
fortheLuMon System
Figure5-4:LuMon Connector
A. LuMon MatchBox connectsto LuMon Belts
B. ControlBox(with integratedelectronics and
statusindicator LED)
C. Monitor plug connects tothe belt connector
socket onthe LuMon Monitor
5.3 Belts
Sentec disposablesingle-patientusebeltsareadhesive-free,embody32electrodesina stripedfabricand
aredesignedtoensureasnugfitbetweenbeltandpatientwithoutrestrictingpatientbreathing.Inparticular,
thebeltsgoaroundthechestfollowingtheribsonaslightlyobliqueplaneforbelts usedonadultsandchildren
oratransverseplaneforbeltsusedon neonatesand infants.Thebeltsmustbeusedonintactskin,are for
single-patientuseandcanbeusedforupto72hours.Donotusebeltsifpackagingisdamaged.Sequential
applicationofSensorBeltsandLuMon Beltsonasinglepatientcanberepeatedforupto30cumulativedays.
CAUTION
DonotuseSensorBeltsorLuMon Beltsifpackagingisdamaged.
5.3.1 SensorBelt
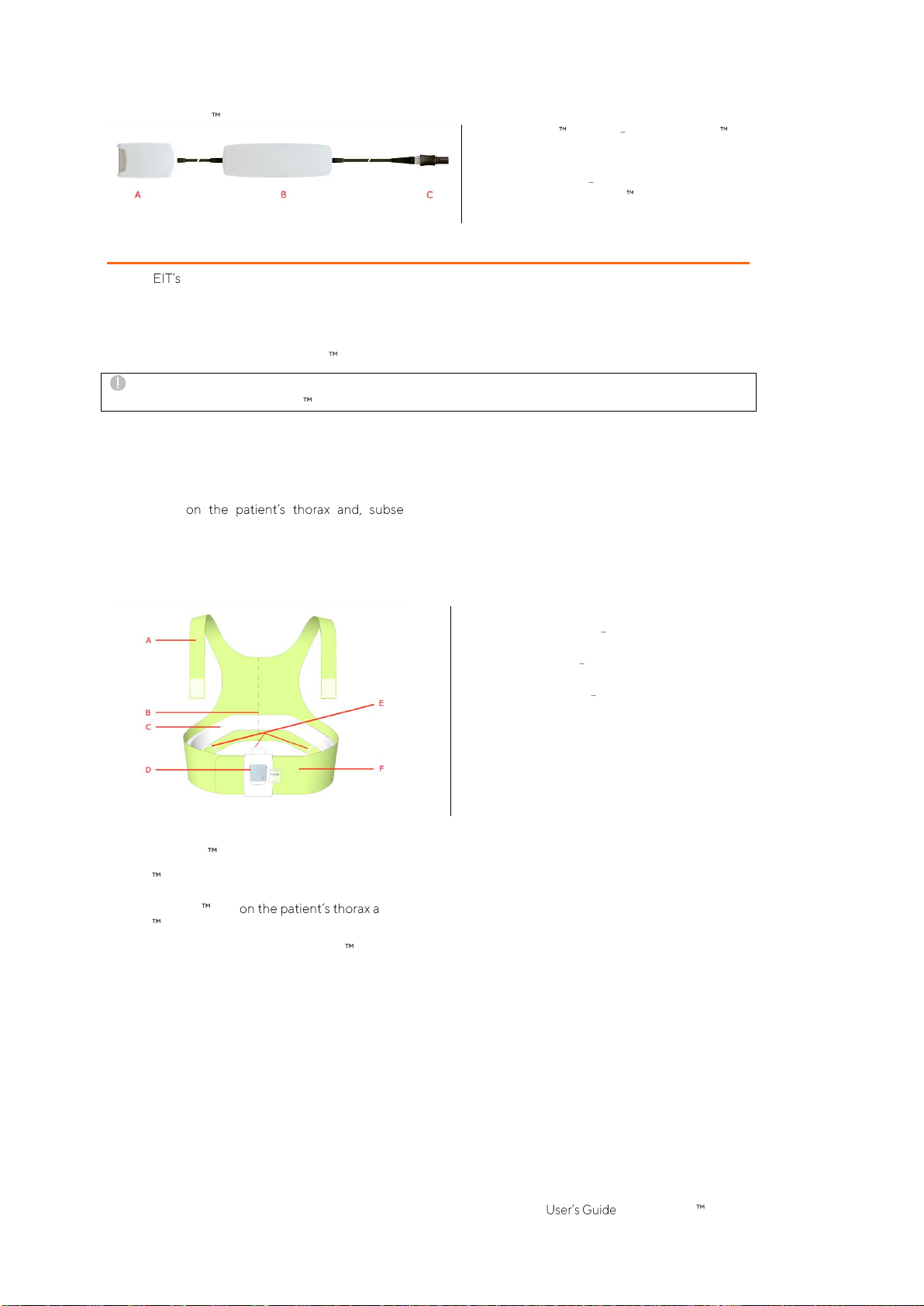
SensorBelts (Figure5-5) are available in four sizes (Table 13-14) and are intended for adults and children whose
full underbust girth is within approximately 76 to 128 cm. Their shoulder straps help to properly position
SensorBelts quently, help to avoid inadvertent displacement of the
SensorBeltfromitsoptimalposition.
The oblique design enables the SensorBelt to follow the movement of the ribs and thus does not restrict
breathing.
Figure5-5:SensorBelt
A. Shoulderstrapswith hookandloopfasteners
B. Mid-line indicator positioning aid (to bealigned
withthespinal column)
C. Stripedfabric adhesive-free,conductive cloth
embedding32 electrodes
D. Dockingstation for connectionofthe
SensorBeltConnector
E. Loopstoattachshoulder straps
F. Flapof SensorBelt
5.3.2 LuMon Belt Adult
LuMon Belts Adult (Figure 5-6) are available in four sizes (Table 13-14) and are intended for adults and
children whose full underbust girth is within approximately 78 to 130 cm. Their shoulder straps help to properly
position LuMon Belt nd, subsequently, help to avoidinadvertent displacement of the
LuMon Beltfromitsoptimalposition.
The oblique design enables the LuMon Belt Adult to follow the movement of the ribs and thus does not
restrictbreathing.
Other manuals for LuMon
1
Table of contents
Other Sentec Medical Equipment manuals
Popular Medical Equipment manuals by other brands

Beijing Choice Electronic Technology
Beijing Choice Electronic Technology MD300W512 manual

Medicatlantic
Medicatlantic XXL user manual

laerdal
laerdal SimMan 3G Maintenance Guide

Siemens
Siemens MULTIMOBIL 5C troubleshooting guide

Hemochron
Hemochron Response Operator's manual

Porter
Porter Matrx MDM installation manual