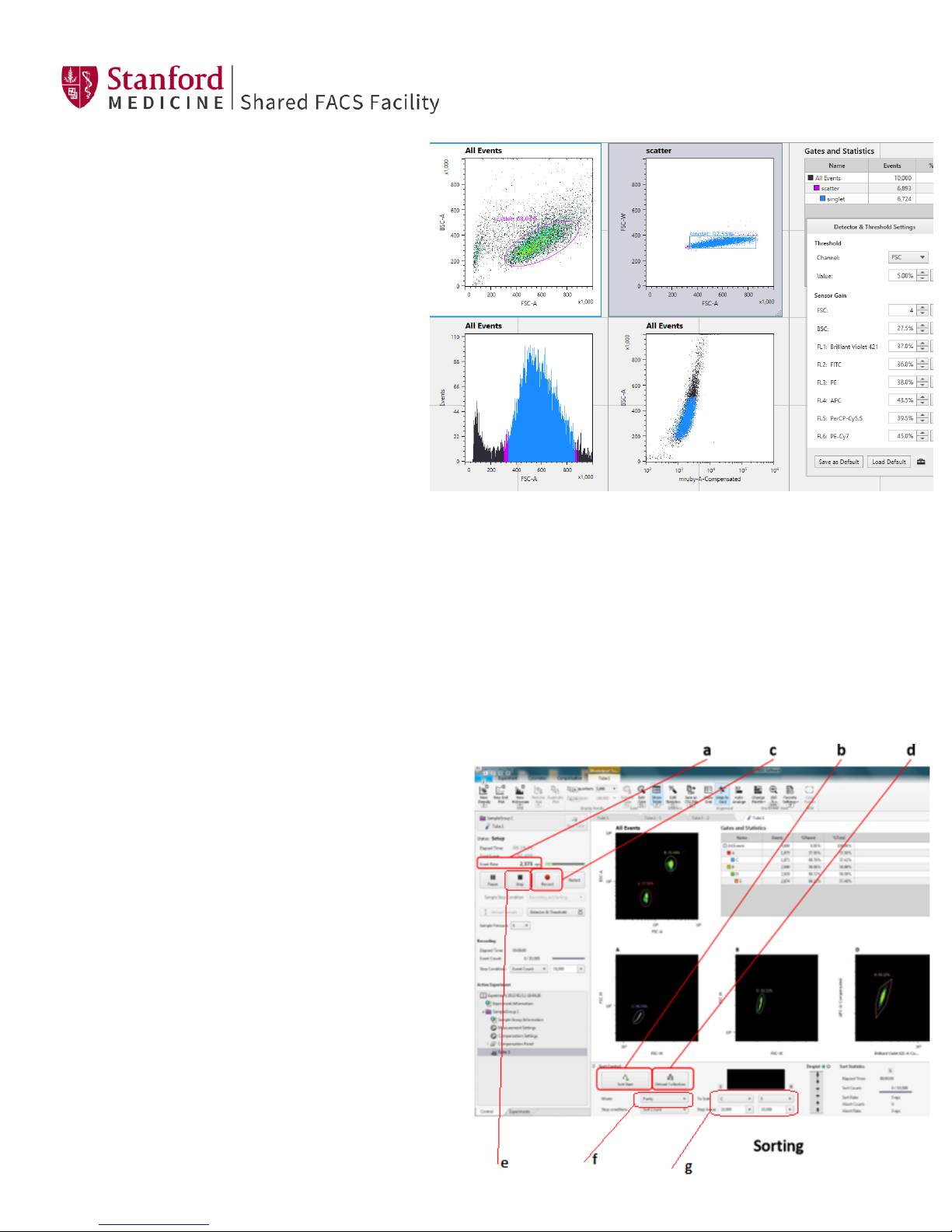
11. To stop the sort, press the black Stop button. (e)
6. Sorting into a Plate
1. Install splash guard, plate stand and plate holder (stored in refrigerator).
2. Change the Sort Method to 96-well plate.
3. Start the sample, then Pause when enough cells are seen to set sorting gates. Pause
the sample and set the gates.
4. Click on Sort Settings to open the 96 well sort setup and plate adjustment.
5. Load a plate with the cover on.
6. Select the Plate Adjustment tab.
7. Select ‘4 corners and center’
8. Click ‘Start’ and the SH800 will sort 50 drops into the corner wells and a center
well.
9. When it finishes, the plate will unload and you can remove it to check the drop
positions over the wells. Note the adjustments needed, and re-load the plate. Click
on each corner one at a time, and adjust the drop position. The default adjustment
of 1 mm can be changed down to 0.1 mm if desired. Once the adjustments are
finished, click ‘Start’ again.
10. Remove the plate to be sure that the adjustments are correct, re-do if necessary.
11. To be sure you will get a nice cell distribution with your actual sample sorting (BSL1 only), verify sorting with the
sample running:
i. In the Plate Sort Settings tab, select a few wells, then the plate settings to sort at least 25 cells of a large
population. Enter values for the sort gate, sort mode, click Add, then close the window.
ii. Keep the cover on the plate and click ‘Sort and Record Start’.
iii. Remove the plate and check to be sure the drops that now have cells inside are centered. If not, go back into sort
settings and re-adjust. If the drops are splattered, the nozzle size is too small for these cells, and you should go to
a larger one.
12. Make a new tube in the experiment (click Next Tube). Click Start, then Pause.
13. Go back into Sort Settings and clear
the wells, then set up the gate and
number of cells per well for your sort.
Check the Index Sort box if desired.
14. Remove the plate lid.
15. Click ‘Sort and Record Start’.
16. When sort is finished, you will have
the option to continue sorting. Click
continue if you wish to sort additional
plates with the same sample. You will
have the opportunity to change sort
criteria. Selecting “no” will require
that you unload/reload the sample.
17. Remove the plate holder (store in refrigerator) and splash guard.
18. To analyze index sort data, click on the Worksheet Tools and ‘Analyze Index Data’
*If the waste needs to be emptied or sheath filled after the auto calibration has already been done, it will need to be
put in standby mode. If you do not put the machine in standby mode and attempt to empty the waste, the stream will
shut off and you will have to run the auto calibration again.