TTaabbllee ooff CCoonntteennttss
Warning/Caution/Note Definition ....................................................................................................................2
Summary of safety precautions......................................................................................................................3
Pinch points...........................................................................................................................................5
Introduction ..................................................................................................................................................6
Product Description................................................................................................................................6
Indications for use ..................................................................................................................................6
Clinical benefits......................................................................................................................................7
Expected service life...............................................................................................................................7
Expected life ...................................................................................................................................7
Disposal/recycle ..............................................................................................................................7
Contraindications ...................................................................................................................................7
Specifications ........................................................................................................................................7
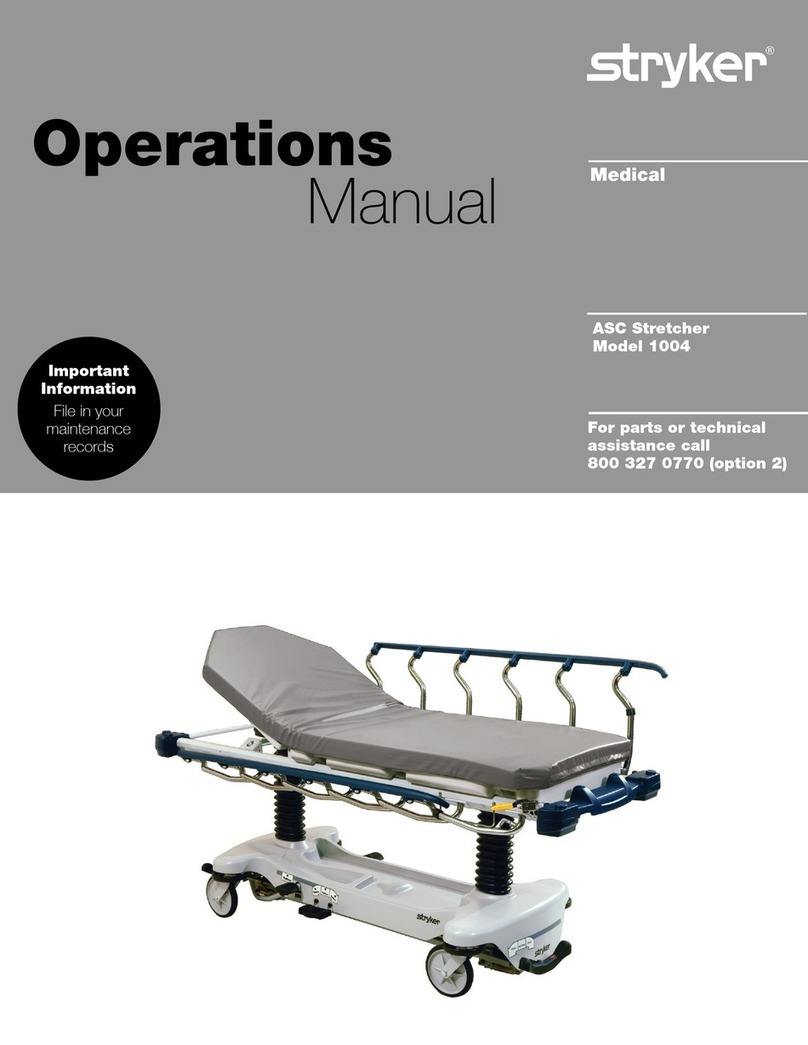
Product illustration .................................................................................................................................9
Applied parts........................................................................................................................................10
Contact information ..............................................................................................................................10
Serial number location..........................................................................................................................11
Serial number location ...................................................................................................................11
Setup.........................................................................................................................................................12
Setup the mattress ...............................................................................................................................12
Operation...................................................................................................................................................13
Applying and releasing the brakes .........................................................................................................13
Base controls.......................................................................................................................................14
Raising the litter ...................................................................................................................................15
Lowering the litter.................................................................................................................................15
Positioning the product in Trendelenburg ...............................................................................................15
Positioning the product in Reverse Trendelenburg ..................................................................................15
Transporting a patient with the retractable fifth wheel ..............................................................................16
Transferring a patient between surfaces.................................................................................................16
Positioning or stowing the head end push handles option ........................................................................16
Positioning or stowing the foot end push handles option ..........................................................................17
Raising the siderail...............................................................................................................................18
Lowering the siderail.............................................................................................................................18
Raising or lowering the Fowler backrest .................................................................................................19
Storing objects in the base hood............................................................................................................19
Positioning the two-stage permanently attached IV pole option ................................................................20
Positioning the three-stage permanently attached IV pole option..............................................................21
Accessories and parts .................................................................................................................................23
Attaching the defibrillator tray/chart holder..............................................................................................23
Attaching and positioning the removable IV pole .....................................................................................24
Attaching the upright oxygen bottle holder ..............................................................................................25
Attaching the paper roll holder...............................................................................................................26
Locating the patient restraint strap tie-ins ...............................................................................................28
Inserting or removing X-ray cassettes ....................................................................................................28
Cleaning ....................................................................................................................................................30
Cleaning the product ............................................................................................................................30
Remove iodine.....................................................................................................................................30
Special instructions ..............................................................................................................................31
Cleaning the mattress...........................................................................................................................31
Disinfecting the product...............................................................................................................................32
Disinfecting the mattress.......................................................................................................................32
Preventive maintenance..............................................................................................................................34
Retractable fifth wheel lubrication ..........................................................................................................35
KK-6300-en Rev 03 1 EN