Indications for Use – his infusion set is designed for subcutaneous infusion of medication (including
insulin) only. It consists of an applicator that contains an adhesive site with cannula and needle, and a
separate extension tubing set with buckle for connection to the site. After insertion of the needle and
cannula, the applicator automatically retracts the needle, providing needle protection. The infusion set
can be used with standard luer lock connectors.
Contraindications – DO NOT use for intravenous (IV) infusions, or to infuse blood or blood products.
Access Site Selection – Choose an area where you have a sufficient layer of subcutaneous tissue.
Avoid areas that may be rubbed by clothing (waistband, etc.), and stay at least 2 inches (5 cm)
away from your belly button. Your healthcare provider can help you with site selection and give you
information about properly caring for your site.
Do Not Reuse: Medical devices require specific material characteristics to perform as intended.
These characteristics have been verified for single use only. Any attempt to reprocess the device
for subsequent re-use may adversely affect the integrity of the device or lead to deterioration
in performance.
WARNINGS
• Never fill the tubing with medication or insulin while the infusion set is connected to your body,
or you could deliver an unintended bolus of medication.
• Ask your healthcare provider to identify proper technique and to help you select and maintain
infusion sites:
»Different people have different amounts of subcutaneous tissue. Ask your healthcare provider
for help to identify the correct soft cannula length for you.
»Improper insertion of the soft cannula and failure to maintain your insertion site and tubing can
result in inaccurate medication delivery as well as irritation and/or infection of the infusion site.
»Check your site regularly to make sure it is still in place. If the tape becomes loose, replace the
infusion set as this may mean that the soft cannula is not completely inserted. If your infusion
site becomes inflamed or infected, replace the infusion set and choose a new site well away
from the previous site.
»Remove all air in the tubing and cannula before starting; infusion or medication delivery will be
delayed as the tubing and cannula fill. Make sure the buckle is fully closed on the site hub or
medication delivery will be interrupted.
»Do not leave your tubing hanging loose where it could accidentally catch on objects and
become disconnected.
• If using this infusion set for insulin delivery:
»If your blood glucose becomes inexplicably high, or an occlusion alarm occurs, check the
tubing and site—make sure the buckle is fully closed, check for clogs or leaks. If you cannot
find the problem, replace your infusion set.
»Develop a plan with your healthcare provider for quickly replacing missed insulin if you
have a problem with the infusion set. You should also have a plan in place for temporarily
disconnecting your infusion set.
»Test your blood glucose level regularly to quickly identify problems, and to make sure whatever
treatment plan you are using has been effective. This is particularly important before you
temporarily disconnect the tubing and when you reconnect.
»Do not change your infusion set just before bedtime. You must be able to test your blood
glucose 1 to 3 hours after insertion to make sure the infusion set is properly inserted and
insulin delivery is working.
CAUTIONS
• Fluid path is STERILE and nonpyrogenic unless the tamper-evident ring is broken and/or the
applicator appears damaged, broken or becomes wet with any liquid.
• Use applicator and tubing set once and then discard.
• * Verify needle retracted. If still visible, press down on OUTER edge until it retracts.
• Tubing set is STERILE and nonpyrogenic unless package is opened or damaged. Do not use if
package is opened or damaged, or if product appears damaged.
• Do not put disinfectants, perfumes, deodorants or lotions on the infusion set or on the infusion
site, as it could cause damage to the product.
• Store your infusion sets in a clean, cool, dry place. Leaving infusion sets in direct sunlight or in
your car may cause damage.
• Observe time and temperature limits for stability of medication. Do not use with substances that
are incompatible with fluid path materials. Make sure medication is at room temperature before
use or air bubbles could form in tubing.
• Carefully read and follow instructions provided with pump, cartridge (if applicable) and medication.
• Dispose of used applicator, needles, tubing, cartridges (if applicable) and medication containers
according to your local requirements.
Materials in the fluid path are medical grade: polycarbonate, silicone, fluorinated ethylene propylene,
urethane/acrylate (uv cured), polyethylene and stainless steel. Replace your infusion set every 48 to 72
hours, according to Centers for Disease Control guidelines, or as your healthcare provider instructs you.
©2012 Smiths Medical. All rights reserved.
Cleo 90 is a registered trademark of Smiths Medical.
Tandem Diabetes Care is a trademark of Tandem Diabetes Care, Inc.
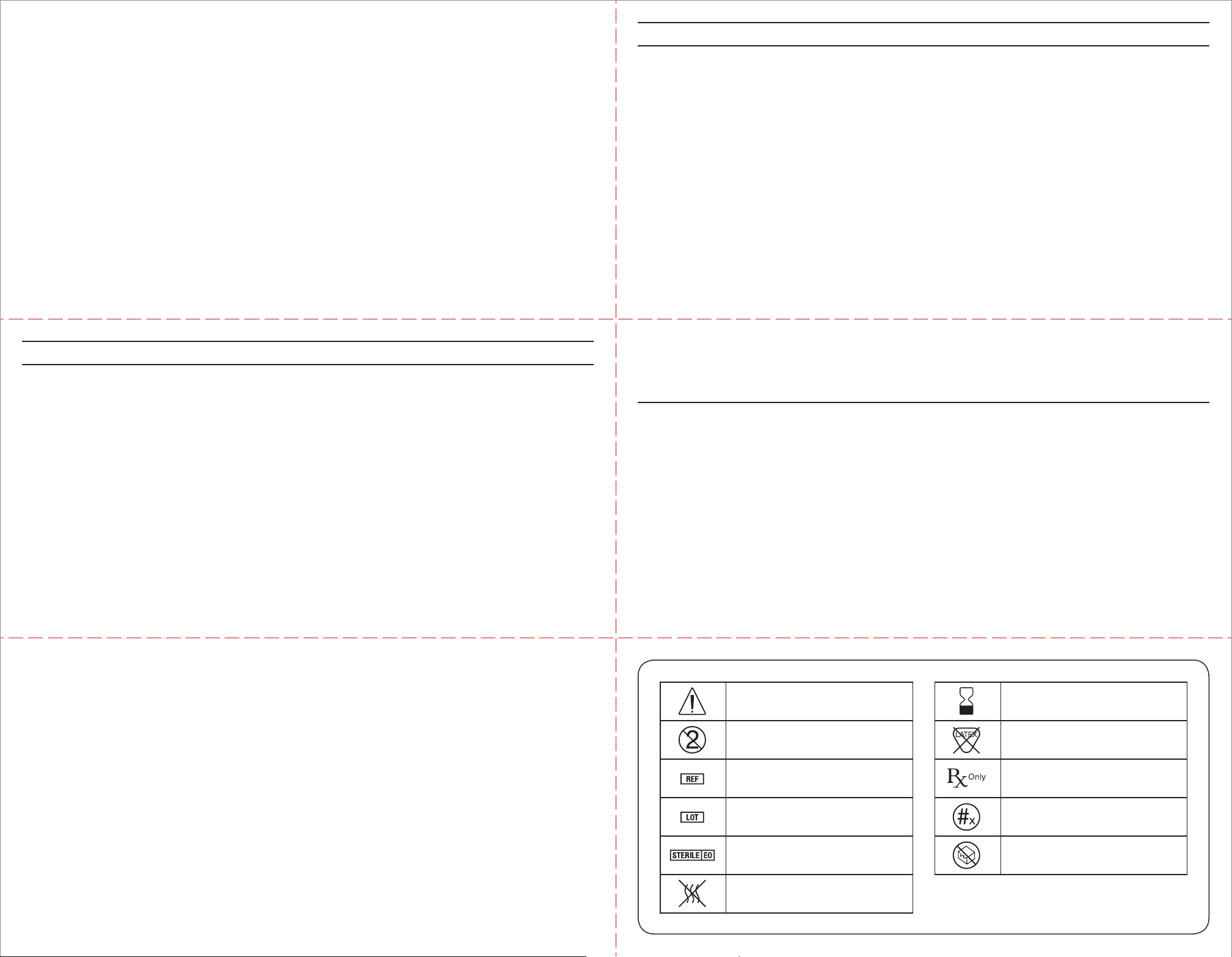
Caution Use by
Do not reuse Does not contain natural
rubber latex
Catalog number
Caution: Federal (USA) law
restricts this device to sale by
or on the order of a physician
Batch code Quantity
Sterilized using ethylene oxide Do not use if package is damaged
Non-pyrogenic

