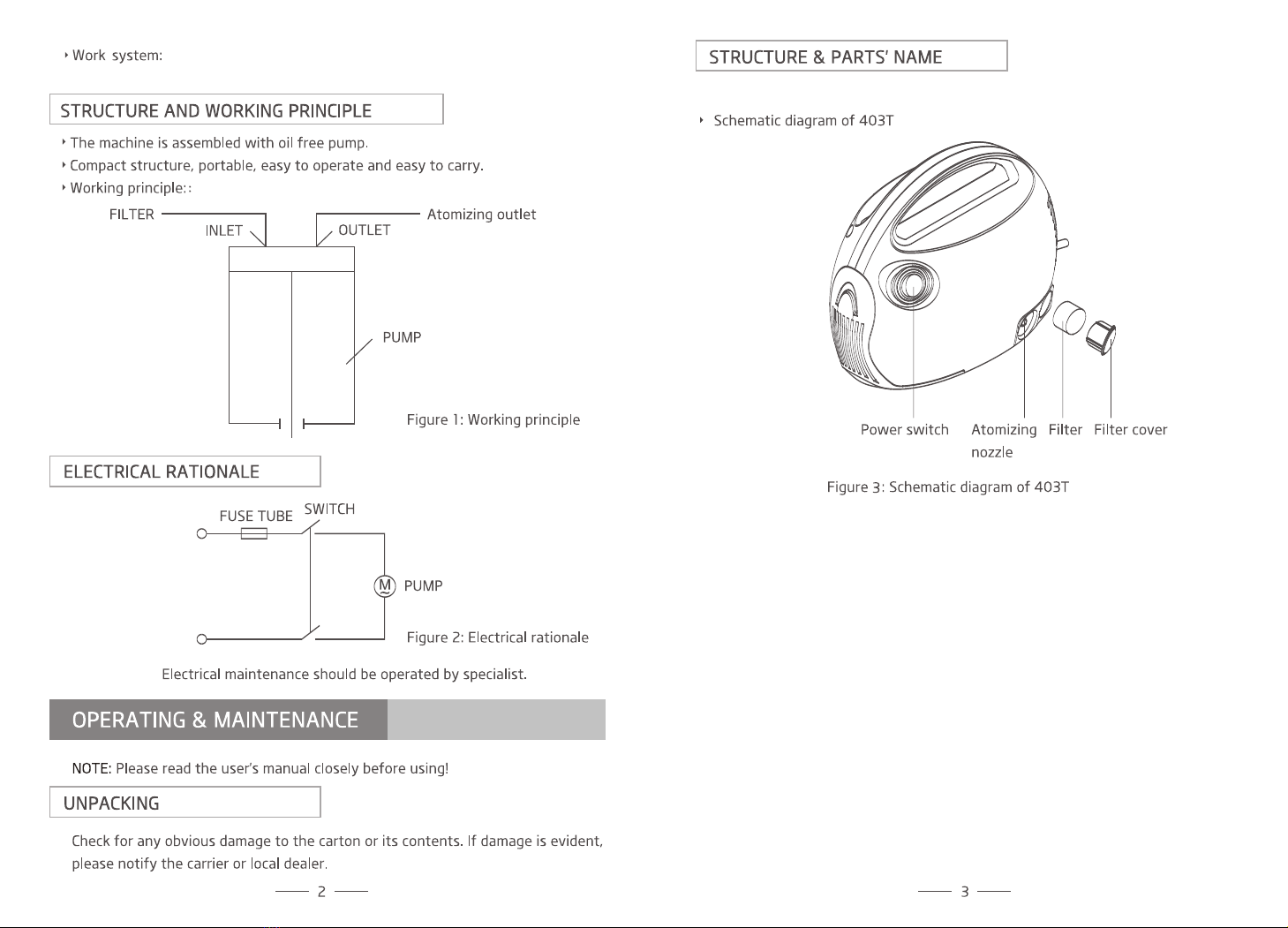
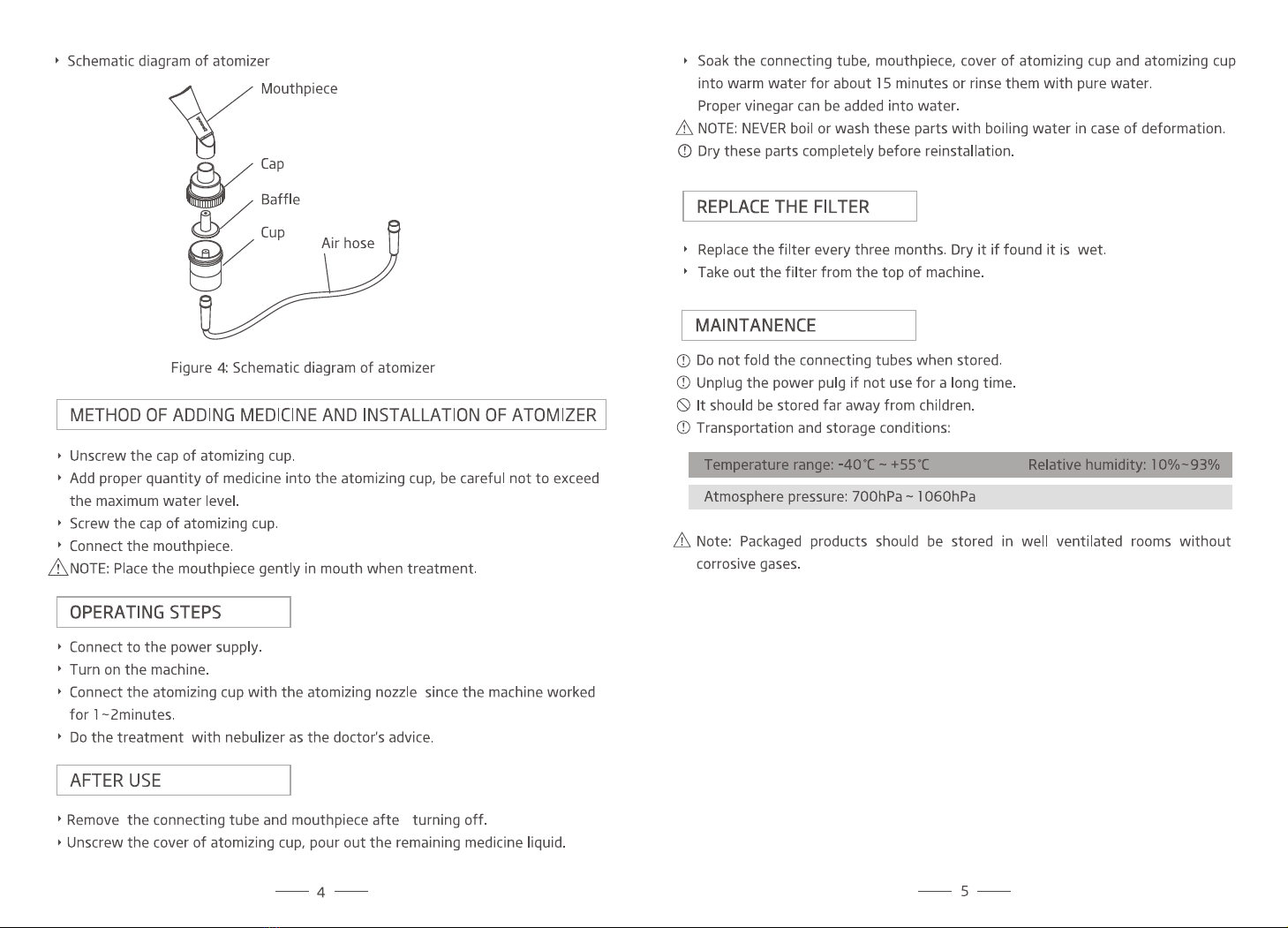
1. Cleaning
Following cleaning instructions after each use will prevent any remaining
medication in the tank from drying, resulting in the device nebulizing fectively
and also will help prevent infections.
First make sure the power switch is in the of position, and then unplug the
power cord from the power outlet. Unplug the tube from the air outlet, remove
the mouthpiece(mask), unscrew the nebulizer kit and discharge remaining
medication in medication tank.
Cleaning
1) Medication tank, mask and mouthpiece
Rinse with clean water or immerse in warm water for 15 minutes to ensure
hygienic cleaning. Add appropriate amount of vinegar in the water, and allow the
parts to air dry in a clean environment.
2) Main unit and air tube
Please wipe with moisturized cloth.
3) Methods for expelling the moisture inside the air tube
a) Make sure the air tube is connected to the main unit.
b) Remove the air tube from the medication tank.
c) Turn on the compressor and pump air through the air tube to expel the
moisture.
2. Disinfecting
Make sure the parts are disinfected properly after each use. If there is pollution
with the parts, please replace with new parts timely. For appropriate methods of
disinfecting, please see the following two instructions:
1) Use a commercially available disinfectant. Please follow the instructions
provided by the disinfectant manufacturer.
A) Disinfectant soaking should be in accordance with the time required in the
instruction manual of disinfectant.
B) Rinse with clean warm water and allow to air dry in a clean environment.
Caution: Never clean with benzene, thinner or flammable chemical.
Rinse the parts with clean hot tap water, and parts may be boiled between
15 to 20 minutes. After boiling, carefully remove the parts, shake off
excess moisture and allow to air dry in a clean environment.
Caution:
Do not boil the air tube, mask, mouthpiece, medication tank, air filter, or
accessories to avoid heat deformation.
After cleaning, all attachments must be dried and then stored in a clean
environment.
Discard any remaining medication in the medication tank.
Please do not left any cleaning fluid residue in the medication tank, and wash
with clean warm water after disinfection.
Always dispose of any remaining medication in the medication tank after each
use. Use fresh medication next time.
Typically, an air filter shall be replaced every three months. If the filter core has
changed color, replace it with a new one.
Air filter Replacement
Caution:
Before use, make sure the air filter is properly installed.
Do not block the air filter.
Use only original air filter designed for this device. Do not operate without a filter.
Do not attempt to wash or clean the filter. If you notice the air filter is wet, please
replace with a new one, otherwise it may cause blockages.
Check that the air filter is clean and free of dust before installation.
Waste and residue Process
The processing of discarded main unit, accessories and other products should be in
accordance with the provisions of the local government.
Notification:
1. For machine damages and failures caused by improper use or failure to follow the
instructions, the company shares no responsibility.
2. When the conditions of temperature, voltage, and product characteristics are not in
line with defined indicators, the main unit may not operate.
3. Product performance may vary with the characteristics of the medication
(suspension or high viscosity).
The company reserves the rights to change the product technology and appearance.
Please don't mind!