Zimmer ThermoPro User manual
Other Zimmer Medical Equipment manuals
Zimmer
Zimmer ZWave User manual

Zimmer
Zimmer Hall MicroChoice System User manual

Zimmer
Zimmer ThermoTK User manual

Zimmer
Zimmer Z Lipo Med User manual

Zimmer
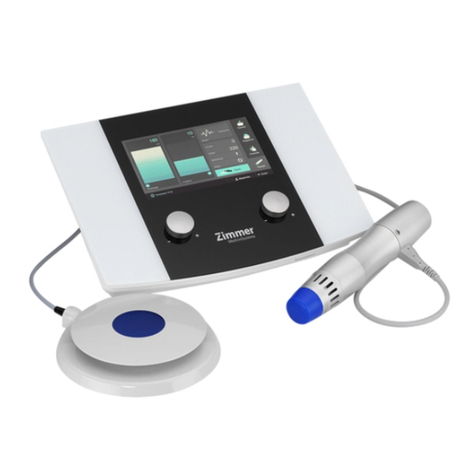
Zimmer OptonPro User manual

Zimmer
Zimmer Cryo 6 User manual
Zimmer
Zimmer Soleo Sono User manual

Zimmer
Zimmer Persona Trabecular Metal Femoral Component User manual

Zimmer
Zimmer Persona User manual

Zimmer
Zimmer enShock User manual

Zimmer
Zimmer Soleo SonoStim User manual

Zimmer
Zimmer SonoOne User manual

Zimmer
Zimmer CITrac User manual

Zimmer
Zimmer enPulsPro User manual

Zimmer
Zimmer A.T.S. 1200 User manual

Zimmer
Zimmer OptonPro User manual

Zimmer
Zimmer re User manual
Zimmer
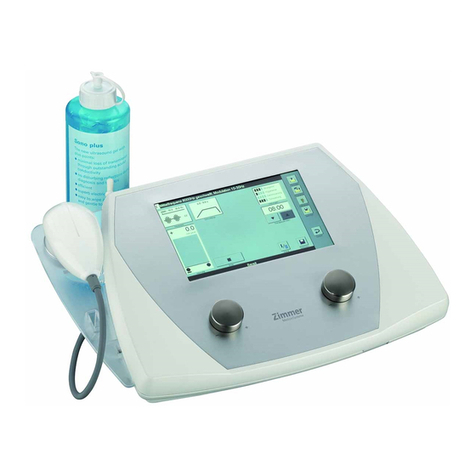
Zimmer Opton User manual

Zimmer
Zimmer OptonPro User manual

Zimmer
Zimmer PremoPortFour User manual
Popular Medical Equipment manuals by other brands

Getinge
Getinge Arjohuntleigh Nimbus 3 Professional Instructions for use

Mettler Electronics
Mettler Electronics Sonicator 730 Maintenance manual

Pressalit Care
Pressalit Care R1100 Mounting instruction

Denas MS
Denas MS DENAS-T operating manual

bort medical
bort medical ActiveColor quick guide

AccuVein
AccuVein AV400 user manual

























