3
8. To activate the biological
indicator, place it in an Attest™
Biological Indicator Activator.
Close and squeeze the activator
to close the 1492VBI cap and
crush the media ampoule. Then
remove the BI and flick it (see
pictures at right). Visually verify
that media has flowed into the
growth chamber at the bottom of
the vial. If the media hasn’t filled
the growth chamber, hold the BI
by the cap and flick it until media
fills the growth chamber. Place
the activated 1492VBI:
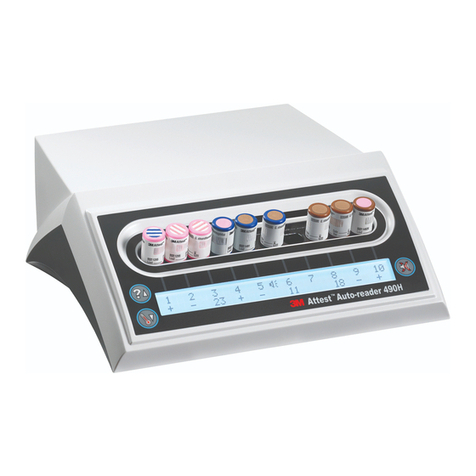
- in any well of a 490or 490H
Auto-reader having software
version 4.0.0orgreater
or
- in an incubation well of a
490Auto-reader having
software version less than
4.0.0which is color-coded
brown (i.e., configured to
incubate 1492VBIs)
and wait for the result. See the
Auto-reader Operator’s Manual
for further information related
to its use.
9. Each day that a processed
1492VBI is incubated, activate
and incubate at least one
non-processed 1492VBI to use
as a positive control. Follow the
activation instructions provided
in Step 8above. Write a "C" (for
"control") and the date on the BI label. The positive control should be from the
same lot code as the processed biological indicator. The positive control BI
helpsconfirm:
• correct incubation temperatures are met,
• viability of spores has not been altered due to improper storage
temperature, humidity or proximity to chemicals,
• capability of media to promote rapid growth, and
• proper functioning of the Auto-reader.
10. Incubation and Reading:
Incubate the positive control and steam processed 1492VBIs in a
490Auto-reader or a 490H Auto-reader having software version 4.0.0or
greater. See the Auto-reader Operator’s Manual for the proper use of this
equipment. The Auto-reader will indicate a positive result as soon as it is
obtained. The final negative 1492VBI reading is made at:
- 24minutes in 490or 490H Auto-readers having software version
4.0.0orgreater
- 1hour in 490Auto-readers having software version less than 4.0.0.
After the results are displayed and recorded, the 1492VBIs may be discarded.
Interpretation of Results
Fluorescent Results
The positive control (unprocessed) 1492VBI must provide a positive fluorescent
result (+ on the 490or 490H Auto-reader LCD display). Processed 1492VBI results
are not valid until the positive control reads fluorescent positive. If the positive
control reads negative (- on the LCD display), check the Auto-reader Operator’s
Manual Troubleshooting Guide. Retest the Auto-reader with a new positive control.
With processed 1492VBIs, a positive (+ on the LCD display) result indicates a
sterilization process failure. A final negative processed 1492VBI reading (- on
the LCD display) after the specified incubation time indicates an acceptable
sterilizationprocess.
Act immediately on any positive results for processed BIs. Determine the cause of
the positive BI following facility policies and procedures. Always retest the sterilizer
and do not use sterilizer for processing loads until three consecutive BI results
arenegative.
Optional Visual pH Color Change Result
The 1492VBI is normally discarded after the fluorescent result has been recorded.
If, however, special studies are desired, 1492VBIs may be further incubated for
a visual pH color change result. After activation and during incubation, the white
Nonwoven Material will absorb the bromocresol purple indicator, the pH-sensitive
indicator dye in the growth media, and appear blue. In the case of the positive
control BI a yellow color change of the growth media and/or Nonwoven Material will
appear within 48hours. Any observation of a yellow color within the vial indicates
a positive result.
In the case of a processed 1492VBI, a media and/or Nonwoven Material color
change from purple to yellow indicates a sterilization process failure. A negative pH
color change result, i.e., media and Nonwoven Material remain purple/blue, can be
assessed at 48hours.
2. To reduce the risk associated with incorrect results:
• Do not place tape or labels on 1492VBI prior to sterilization or incubation.
• Do not incubate a 1492VBI if, after processing and before BI activation, it
is observed to have a broken media ampoule. Retest the sterilizer with a
new biological indicator.
• After processing, allow BI to cool for 10minutes before incubation.
• After BI activation, ensure media has flowed to the spore growth chamber.
Monitoring Frequency
Follow facility Policies and Procedures which should specify a biological indicator
monitoring frequency compliant with professional association recommended
practices and/or national guidelines and standards. As a best practice and to
provide optimal patient safety, 3M recommends that every steam sterilization load
be monitored with a biological indicator in an appropriate Process Challenge Device
(PCD i.e., BI challenge test pack).
Directions forUse
1. Identify the 1492VBI by writing the load number, sterilizer, and processing
date on the indicator label. Do not place another label or indicator tape on the
vial or on the cap.
2. Place the 1492VBI in a representative tray configuration or Process Challenge
Device (PCD) as recommended by professional association guidelines or
national standards for healthcare facility practice. Do not place the 1492VBI
in direct contact with a chemical indicator as residue could transfer to the
biological indicator and affect the result.
3. Place the PCD in the most challenging area of the sterilizer. This is typically on
the bottom shelf, over the drain, however, the sterilizer manufacturer should be
consulted to identify the area of the chamber least favorable to sterilization.
4. Process the load according to recommended practices.
5. After completion of the cycle take the PCD out of the sterilizer, and remove
the 1492VBI.
6. Allow the 1492VBI to cool for 10minutes prior to activation.
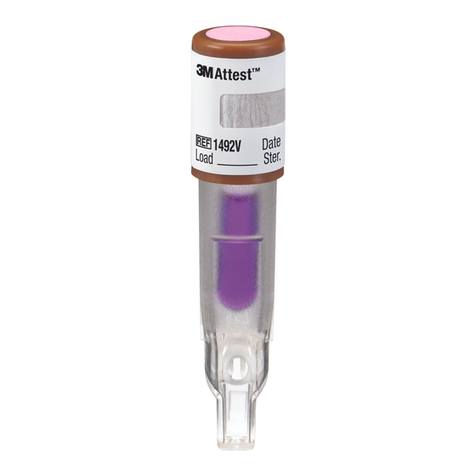
7. Check the process indicator on the top of the cap of the 1492VBI. A color
change from pink to light brown or darker confirms that the 1492VBI has been
exposed to the steam process. This color change does not indicate that the
steam process was sufficient to achieve sterility. If the process indicator is
unchanged, check the sterilizer physical monitors.