Tableof Contents
Explanation of Symbols....................................................... 2
Product and package labels andpictograms �������������������������2
Explanation of Symbols: OperatorManual������������������������������3
ContentDisclaimers ........................................................... 3
1.Description .................................................................... 4
2. IntendedUse.................................................................. 5
3.Safety ............................................................................ 6
4. Dangers and FirstAid..................................................... 7
4�1�Dangers���������������������������������������������������������������������������7
4�2� FirstAid����������������������������������������������������������������������������8
5.Warnings ....................................................................... 9
6.Cautions ...................................................................... 10
7.Specifications............................................................... 11
7�1� 3M™Steri‑Vac™ Sterilizer/Aerator GSX Series
StructuralSpecifications������������������������������������������������11
7�2� Sound Power Levels Specifications�������������������������������11
7�3� PowerSpecifications�����������������������������������������������������11
7�4� Air SupplySpecifications �����������������������������������������������12
8. Compliance and ReferenceStandards .......................... 12
8�1� Device SafetyCompliance���������������������������������������������12
8�2� Electromagnetic Compatibility (EMC)Compliance���������12
9. Installation and SetUp.................................................. 13
9�1� Environmental OperatingConditions �����������������������������13
9�2� Room and InstallationRequirements�����������������������������14
9�3� Set up andConnections ������������������������������������������������15
10. Using the TouchScreen.............................................. 18
10�1� MainScreen����������������������������������������������������������������18
10�2�Menu���������������������������������������������������������������������������18
10�3�Reports������������������������������������������������������������������������19
10.3.1. CycleReports......................................................19
10.3.2. Ethylene Oxide UsageReports..............................20
10.3.3. Site SetupReport ................................................21
10.3.4. Printer FormFeed................................................22
10�4� CycleCategories����������������������������������������������������������22
10.4.1. OperatorCycles...................................................22
10.4.2. SupervisorCycles................................................23
10�5� SetupMenu�����������������������������������������������������������������24
10.5.1. SiteSetup...........................................................24
10.5.2. UserSetup..........................................................28
10�6�Status��������������������������������������������������������������������������30
10.6.1.Control................................................................30
10.6.2.Info.....................................................................30
10.6.3.Log.....................................................................30
11. 3M™ CycleProgrammer........................................... 31
11�1� 3M™ Cycle ProgrammerOverview ����������������������������31
11�2�3M™ Cycle Programmer Hardware and
SoftwareRequirements�����������������������������������������������31
11.2.1. HardwareRequirements ......................................31
11.2.2. SoftwareRequirements .......................................32
11�3�Installing the 3M™ CycleProgrammer�����������������������32
11�4�Creating a Cycle in the 3M™ CycleProgrammer�������34
11�5�Defining Set Points for Cycle Stages andParameters �37
11.5.1. PreheatStage .....................................................37
11.5.2. Air RemovalStage...............................................37
11.5.3. Chamber TestStage ............................................37
11.5.4. ConditioningStage ..............................................38
11.5.5. EO InjectionStage...............................................39
11.5.6. EO ExposureStage..............................................40
11.5.7. EO RemovalStage...............................................40
11.5.8. FlushingStage ....................................................41
11.5.9. AerationStage ....................................................42
11.5.10. Save a CustomCycle.........................................42
11.5.11.StandbyStage...................................................43
11.5.12.Estimated Total CycleTime................................43
11�6�Importing Custom Cycles to the 3M™Steri‑Vac™
Sterilizer/Aerator GSXSeries���������������������������������������44
11�7� Running Custom Cycles on the 3M™Steri‑Vac™
Sterilizer/Aerator GSXSeries���������������������������������������47
11�8� Managing Custom Cycles on the 3M™Steri‑Vac™
Sterilizer/Aerator GSXSeries���������������������������������������48
11�9�Replacing Cycles on the 3M™Steri‑Vac™
Sterilizer/Aerator GSX Series With the SameName ����49
11�10�Removing Custom Cycles from the 3M™Steri‑Vac™
Sterilizer/Aerator GSXSeries�������������������������������������50
11�11� CycleReports ������������������������������������������������������������51
11.11.1.Custom Cycle SetPoints....................................51
11.11.2.Export CycleData..............................................52
12. Medical Device Packaging andLoading...................... 53
12�1� Preparing Medical Devices forSterilization �����������������53
12�2� Packaging MedicalDevices�����������������������������������������53
12.2.1. RecommendedPackaging ...................................53
12.2.2. Non‑compatiblePackaging ..................................54
12.2.3. Package MedicalDevices ....................................54
12�3� Loading the 3M™Steri‑Vac™ Sterilizer/Aerator
GSXSeries�������������������������������������������������������������������54
12.3.1. LoadingRecommendations..................................55
12.3.2. Loading Medical Devices andInstruments............55
13. OperatingInstructions ................................................ 56
13�1� Starting aCycle �����������������������������������������������������������56
13�2� Display ScreenIndications ������������������������������������������65
13�3� Overview of GSX Series Ethylene Oxide
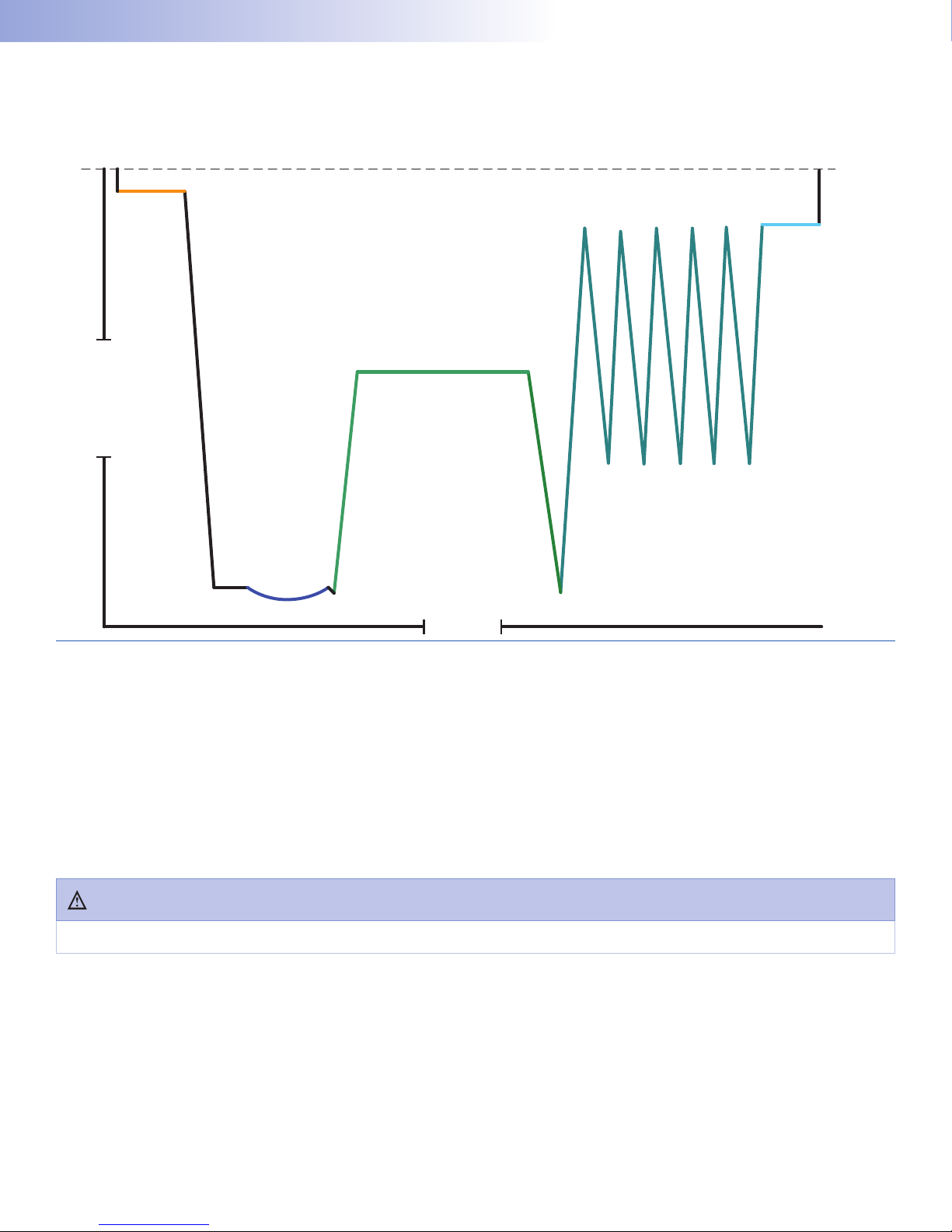
(EO)SterilizationCycle�������������������������������������������������66
13.3.1. Cycle Stages andDescriptions.............................66
13.3.2. CycleReports......................................................67
13�4�Cartridge Dispose Cycle for 3M™Steri‑Gas™
EOGasCartridges �������������������������������������������������������70
13�5�EthernetConnection����������������������������������������������������70
13.5.1.NetworkConnections ..........................................70
13.5.2.IPAddresses.......................................................70
13.5.3.SoftwareSecurity ................................................71
13.5.4.SoftwareUpdates................................................71
13.5.5.FirmwareUpdates ...............................................71
13�6�Distilled WaterReservoir ���������������������������������������������72
13�7�PrinterOverview����������������������������������������������������������72
13�8�Unloading the 3M™Steri‑Vac™ Sterilizer/Aerator
GSXSeries�������������������������������������������������������������������72
13.8.1. Unloading the 3M™Steri‑Vac™ Sterilizer/Aerator
GSX Series – CycleComplete ..............................73
13�9� Accessing the Chamber – Aeration NotComplete�������74
13�10� Empty 3M™Steri‑Gas™ Ethylene Oxide
(EO)GasCartridges ���������������������������������������������������75
13�11� Aeration of a Biological Indicator Process
ChallengeDevice (BIPCD)�����������������������������������������75
13�12�Sterilization CycleCancellations��������������������������������75
13.12.1. Manual CycleCancellation.................................75
13.12.2. Automatic CycleCancellation.............................75
13�13�PowerOutages����������������������������������������������������������75
14. Process Monitoring and LoadRelease ........................ 76
14�1�Physical Parameters andRequirements����������������������76
14�2� Biological Indicators and Process ChallengeDevices��78
15. RoutineMaintenance ................................................. 79
15�1� DailyCleaning �������������������������������������������������������������79
15�2� Air Supply LineFilters��������������������������������������������������79
15�3� PreventativeMaintenance�������������������������������������������80
16. Cautions, Error Messages, andTroubleshooting.......... 81
16�1� CautionMessages�������������������������������������������������������81
16�2� ErrorMessages �����������������������������������������������������������82
16�3� Error Levels and CorrectiveActions�����������������������������83
17. Repair andReplacement............................................ 85
18. PreventativeMaintenance .......................................... 85
19. Ordering Accessories andSupplies............................. 86
20. 3M™ Cycle Programmer Support
and SoftwareUpdates................................................ 87
ContactInformation��������������������������������������������������������������87
U�S� OrderingInformation�����������������������������������������������������87
Orders for Supplies (e�g� 3M™Steri‑Gas™ EO Gas
Cartridges, 3M™ Attest™ Biological Indicators, 3M™
PrinterPaper)*����������������������������������������������������������������������87