10
- USER MANUAL
WARNINGS
Keep away from children or people with mental disabilities.
The device contains small pieces that can be swallowed.
In order to prevent any risk of strangulation, please keep
the electrodes wires away from children.
The device is meant for personal use. For reasons of
hygiene, the electrode should only be used on one person.
Do not use the device by driving or by working on a
machine.
Do not use this electro stimulation unit in a damp
environment, a bathroom, a sauna or close to electric
machinery connected to mains and earthed, or even
piping earthed.
Use this device in a clean place (without dust nor dirt…).
The simultaneous connection of a patient to a high
frequency surgical unit may cause burns at the points of
contact of the stimulator’s electrodes and the stimulator
may be damaged.
Operation in close proximity of a short wave therapy unit
may cause instability in the stimulator’s output power.
Do not, under any circumstances, attempt to open or
repair the Multisportpro yourself! Only have repairs carried
out by the customer service department.
TROUBLE SHOOTING
If your device doesn’t work properly, do not use it and contact
your reseller.
PRECAUTIONS OF USE
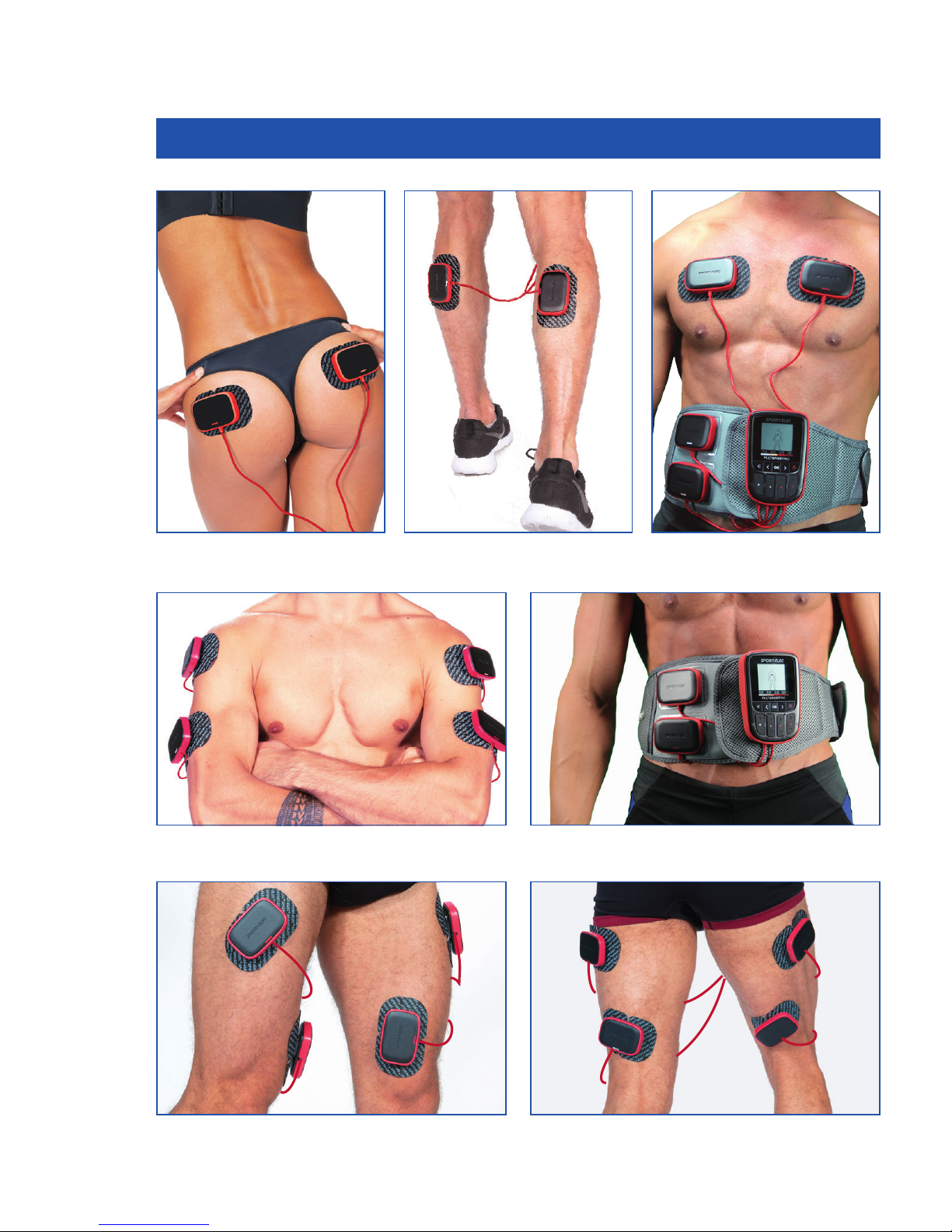
Do not use the unit on your face.
Do not leave within reach of children.
Ask for advice from your physician:
if you have electronic or metallic implants (pins…),
in case of skin condition (wounds…).
CONTRAINDICATIONS
Do not use SPORT-ELEC®:
On the heart area.
If you carry a pacemaker.
If you are pregnant.
During first 6 weeks after baby birth.
In case of neuromuscular diseases.
In case of hemorrhagic diseases.
On the face and skull.
RECOMMENDATIONS FOR MAINTAINING
AND CLEANING
Clean the unit with a tissue dampened with a disinfecting
solution diluted with water at 1/10th.
V. TECHNICAL SPECIFICATIONS
BELT
References : CTBB2MEAV2
Size (unisex) : from 34 to 60
Length of the unfolded belt :122 cm
Fabric : 35% sponge, 20% elastic
fabric, 15% polyester, 15% nylon, 10%
PVC + copper, 2% rubber, 3% non-
woven fabric
ADHESIVE ELECTRODES
Reference : EASF110x71
Contact area : 67.5 x 47 mm (x2)
Estimated life expectancy : 40 uses
Class I medical device (Directive
93/42/CEE)
Adhesive, hydrophilic and
hypoallergenic electrodes
Please order new compatible
adhesive electrodes on www.sport-
elec.com.
AC ADAPTOR
Reference : MN-A403-E120
Input : 100-240V, 50/60Hz, 0.25 A max
Output : 5V 0.6A
The adapter is part of the equipment.
Only use the AC adaptor supplied
with the device.
Please replace this adaptor should it
fail (contact SPORT-ELEC® or order at
www.sport-elec.com).
UNIT
Class IIa medical device in accordance with Directive 93/42/CEE
Software version
........................................................................................
E
Type of current
..........................................................................................
Dual phase
Number of programs
.................................................................................
88 “SPORT” programs - 6 “HEALTH” programs
Number of program variations (or phases)
...............................................
344
Number of adjustable independent outputs
...........................................
4
Maximum intensity on a 500 Ω load/channel
..........................................
100 mA / 500 Ω / channel
Frequency range
.......................................................................................
0.25 to 160 Hz
Pulse width range
......................................................................................
70 to 360 μs
Power supply
.............................................................................................
LiPo Rechargeable battery 3.7V - 1020 mAh
Unit dimensions and weight
.....................................................................
83.5 x 125.5 x 29.61mm - 340g
Automatic stop at end of each program
..................................................
Yes
Activation safety
........................................................................................
100%