(800) 979-2020 US & CA • (610) 889-0200 International • +31 (0) 485-350300 Europe • www.accutome.com 3
Information
The section lists:
• Specic safety precautions
associated with the PachPen
• General safety precautions
Safety Issues to Consider When
Using the PachPen
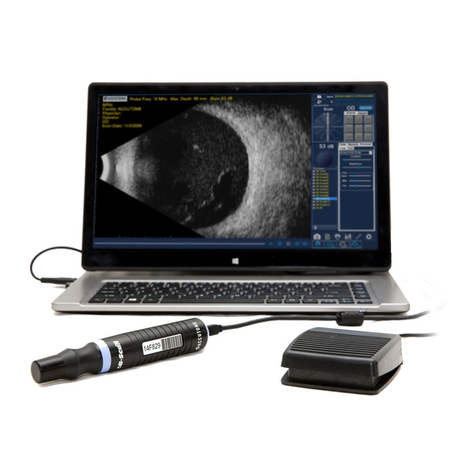
The PachPen is noninvasive. The
ultrasonic biometry probe touches the
surface of the anesthetized cornea
during the scanning process.
Indications for Use
This instrument is used for measuring
the corneal thickness of the eye. It is
to be used in a medical setting and
only by technicians, optometrists, and
ophthalmologists who are experienced
in ultrasonic biometric techniques.
CAUTION: General indications for use of
the PachPen include external, structurally
intact areas of the eye globe and orbit only.
Disposal Requirements
Disposal of the product within
the EU
The PachPen contains electronic
components. At the end of its useful
life, it must be properly disposed of in
compliance with local regulations.
EU directives and national regulations
currently in force at the time of
marketing prohibit the disposal
of the PachPen specied on the
delivery note in domestic waste or by
municipal waste disposal companies.
If the PachPen or its components
are resold, the seller has the duty to
notify the buyer that the product must
be disposed of in accordance with
currently valid national regulations.
Symbol Definitions for the
PachPen
Statements, graphics, and symbols
listed below are used on components
of the PachPen. Descriptions and
meanings are listed to the right of the
symbols.
Chapter 2: Safety
2Safety
Attention! Consult
Instruction Manual
Action Control
Button
Battery
Replacement
Type B Medical
Device
Class II Insulation
Disposal of Product
within the EU