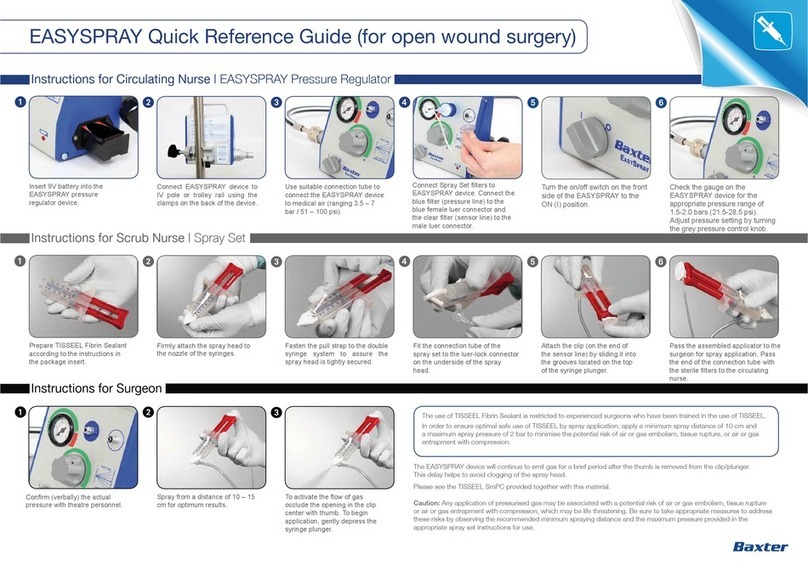
CAUTION BOX
Any application of pressurised gas may be associated with a potential risk of air or gas embolism, tissue rupture or air or gas entrapment with
compression, which may be life threatening if the product is sprayed incorrectly
Precautions
For TISSEEL/TISSUCOL Fibrin Sealant
• Spray application should only be used if it is possible to accurately judge the spray distance as recommended by the manufacturer.
Do not spray closer than the recommended distances.
• Prior to applying sprayable brin solutions for sealant, the surface area of the wound should only be dried using standard techniques
(eg, intermittent application of compresses, swabs, use of suction devices).
• Blood pressure, pulse rate, oxygen saturation and end tidal CO2should be monitored closely when spraying fibrin solutions for sealant using a
pressure regulator device, because of the possibility of occurrence of air or gas embolism.
• Regulators should be used in line with manufacturer recommendations and the SmPC and Instruction for Use.
• In laparoscopic procedures: Use CO2only when using spray application. When applying the product as a spray using a CO2pressure regulator device,
the maximum pressure should be 1.5 bar (22 psi/maximum gas flow rate 2.0 liters per minute). The product should be sprayed at a distance at least 2 cm
(recommended range 2-5 cm) from the tissue surface.
Recommended pressure, distance and devices for spray application of TISSEEL
Surgery Spray set to be used Pressure regulator
to be used Pressure regulator to be used Recommended distance
from target tissue
Recommended spray
pressure
Laparoscopic/ minimally
invasive procedures n.a.
DUPLOSPRAY
MIS Applicator
20cm
DUPLOSPRAY MIS Regulator
2 – 5 cm 1.2-1.5 bar (18-22 psi)
Duplospray MIS Regulator NIST B11
DUPLOSPRAY
MIS Applicator
30cm
Duplospray MIS Regulator
Duplospray MIS Regulator NIST B11
DUPLOSPRAY
MIS Applicator
40cm
Duplospray MIS Regulator
Duplospray MIS Regulator NIST B11
Replaceable tip
Duplospray MIS Regulator
Duplospray MIS Regulator NIST B11
Connect to regulated CO2gas source only; maximum input pressure not to exceed 100 psi ± 5 (6.89 bar ± .35). To avoid possible air or gas embolism, do not directly
spray into circulatory pathways.
Caution: Any application of pressurized gas may be associated with a potential risk of air or gas embolus, tissue rupture or air or gas entrapment with compression,
which may be life threatening. Be sure to take appropriate measures to address these risks by observing the recommended minimum spraying distance of 2 cm (optimal
working distance 3 cm) and the maximal flow rate of 2.0 standard liters per minute.
In order to ensure optimal safe use of TISSEEL by spray application the following recommendations should be followed:
When spraying the TISSEEL, changes in blood pressure, pulse, oxygen saturation and end tidal CO2should be monitored because of the possibility of occurrence of air
or gas embolism.
For detailed information please contact your local representative.
In some countries TISSEEL Fibrin Sealant is licensed under the trademark of TISSUCOL Fibrin Sealant.
Baxter, Tisseel, Tissucol, and Duplospray are trademarks of Baxter International Inc., its subsidiaries or afliates.
July 2015 ROI/182/15-0001
Baxter Healthcare Ltd.
Unit 7 Deansgrange Business Park
Blackrock
Co. Dublin
Tel: +353 1 2065500
Fax: +353 1 2065555
www.baxterhealthcare.ie
b
DUPLOSPRAY Quick Reference Guide
PRESCRIBING INFORMATION - TISSEEL
Lyo Two-Component Fibrin Sealant
Ready to use Solutions for Sealant
(Please consult the Summary of Product Characteristics before prescribing)
Name and composition: : Tisseel Lyo - powders and solvents for fibrin sealant. 1) Sealer protein
concentrate, after reconstitution 1 ml contains 91 mg Human Fibrinogen (as clottable protein); 0.6-5 IU
Human Factor XIII and 3000 KIU Aprotinin; 2) Thrombin solution, after reconstitution, 1 ml contains 500
IU of Human Thrombin and 40μmol Calcium Chloride.
Tisseel Ready to use – prefilled double chamber syringe containing Sealer Protein Solution (with
aprotinin) deep frozen in one chamber and Thrombin Solution (with Calcium Chloride) deep frozen in the
other chamber. Sealer Protein Solution contains 91mg/ml Human Fibrinogen (as clottable protein), 0.6-
5 IU/ml Factor XIII and 3000 KIU/ml Aprotinin. Thrombin Solution contains 500 IU/ml Human Thrombin
and 40μmol/ml Calcium Chloride. Presentations of 1, 2 or 5ml in each chamber resulting in total volume
of 2ml, 4ml or 10ml of sealant.
Indications: As a coagulant producer for use as a tissue sealant and haemostatic, for surgical incisions,
plastic surgical repairs, orthopaedic, traumatic, and dental surgery. Dosage and Route: The use of
TISSEEL is restricted to experienced surgeons who have been trained in the use of TISSEEL. A thin
layer is applied to the tissue surface where required. Dose depends on the indication, application
method and number of applications. As a guideline for the gluing of surfaces, 1 pack of TISSEEL 2 ml
(i.e. 1 ml Sealer Protein Solution plus 1 ml Thrombin Solution) will be sufficient for an area of at least 10
cm2. Apply topically – tissue surface should be as dry as possible before application. Application can be
repeated if necessary. Apply by drops or spray as needed depending on indication. Safety and efficacy in
paediatric population not established. Side effects: See Summary of Product Characteristics for detail.
Hypersensitivity / anaphylactic / anaphylactoid reactions may occur, especially in patients who have
previously received aprotinin. Early symptoms of allergic reactions include flushing, urticaria, pruritus,
nausea, hypotension, tachycardia or bradycardia, bronchospasm, tightness in chest and dsypnoea.
Postoperative wound infection, fibrin degradation products increased, paresthesia, wheezing,
erythema, bradycardia, tachycardia, auxillary vein thrombosis, hypotension, haematoma, cerebral
artery embolism, cerebral infarction, intestinal obstruction, impaired healing, procedural pain, flushing,
oedema, angioedema, sensory disturbance, pain, nausea, increase in body temperature, rash, pain in
extremity and seroma have been reported. Do not inject – risk of thromboembolic complications. Risk
of arterial embolism. Precautions: Apply with care in coronary artery bypass surgery due to increased
risk of inadvertent intravascular application. TISSEEL and/or Thrombin Solution should only be applied
topically. Do not inject in soft tissue – risk of local tissue damage. When spraying TISSEEL, changes
in blood pressure, pulse, oxygen saturation and end tidal CO2should be monitored because of the
possibility of occurrence of air or gas embolism. See SmPC for further details. Air or gas embolism,
tissue rupture, or gas entrapment with compression, which may be life-threatening or fatal, have
occurred with the use of spray devices with air or gas employing a pressure regulator to administer fibrin
sealant. These events appear to be related to the use of the spray device at higher than recommended
pressures and in close proximity to the tissue surface. Must not be used with Easyspray/spray set in
enclosed areas. When applying by spray, follow the instructions provided with the spray device, with
particular reference to gas pressure and distance from the tissue surface. Use with caution in patients
with prior exposure to aprotinin. Caution in patients with bovine protein allergies. Infectious diseases
due to the transmission of infective agents cannot be totally excluded. Use of Tisseel and batch number
should be recorded in patient‘s notes. Excessive clot thickness may negatively interfere with product
efficacy and the healing process. Oxidised cellulose-containing preparations should not be used with
Tisseel. The effect of Tisseel on fertility has not been established. Contraindications: Do not apply
intravascularly – can be life threatening. Hypersensitivity to active substances or other components. Not
for the treatment of massive and brisk arterial or venous bleeding. Do not use to replace skin sutures
intended to close surgical wounds. Interactions: No formal interaction studies have been performed.
Thrombin component may be denatured by alcohol, iodine or heavy metals (e.g. antiseptic solutions).
Overdose: Not reported. Legal category: POM Marketing Authorisation Number and Holder:
TISSEEL Lyo - PA 167/129/6. TISSEEL Ready to use - PA 167/129/005 Baxter Healthcare Limited,
Caxton Way, Thetford, Norfolk IP24 3SE Date of preparation: May 2014
Further information is available on request.
Reporting suspected adverse reactions after authorisation of the medicinal product
is important. It allows continued monitoring of the benefit/risk balance of the medicinal
product. Healthcare professionals are asked to report any suspected adverse reactions via
HPRA Pharmacovigilance, Earlsfort Terrace, IRL-Dublin: Tel:+353 1 6764971; Fax: +353 1
Adverse events should also be reported to Baxter Healthcare at +353 (0) 1 2065500,
or by contacting your local Baxter representative.