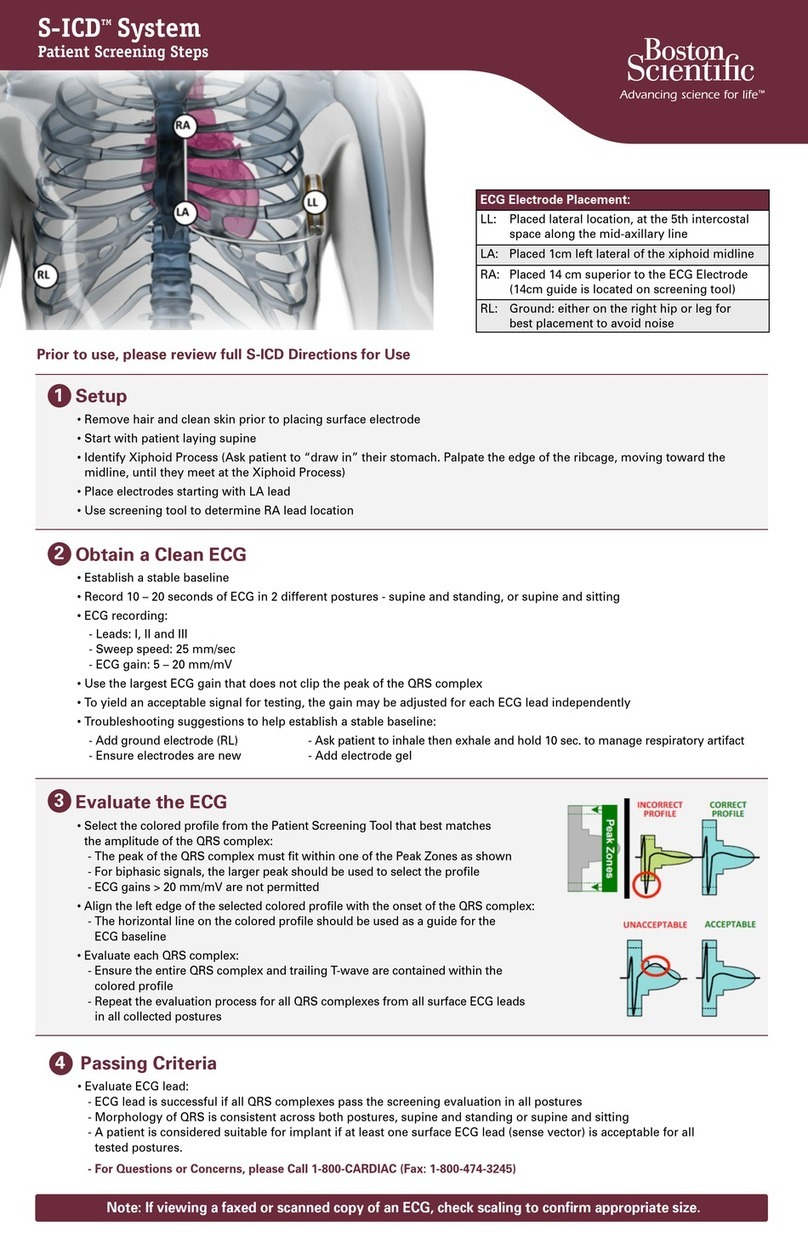
5
Boston Scientific (Master Brand DFUTemplate 8.5in x 11in Global, 90106042 RevAN), MB, AngioJet Ultra System Operators Manual, Global, 91024738-01C
Black (K) ∆E ≤5.0Black (K) ∆E ≤5.0
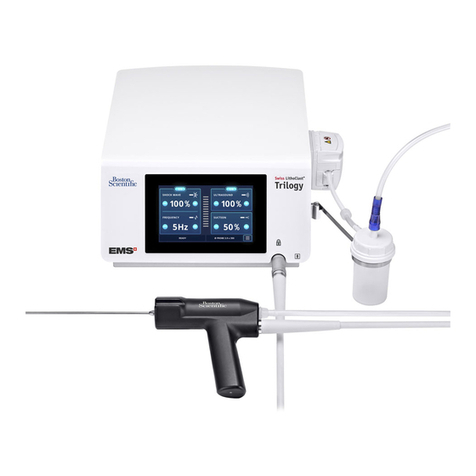
The AngioJet™ Ultra Thrombectomy Set
(Thrombectomy Set - Several models available)
The single-use, disposable Thrombectomy Set includes:
• Catheter
• Pump
• Salinedeliverytubingandwastetubing
• Collectionbag
Figure 2. Thrombectomy Set
A bag of sterile, heparinized saline (not included) supplies the pump with
saline through the saline delivery tubing. The pump pressurizes the saline. The
Thrombectomy Set uses this pressurized, high-velocity saline to create a low-
pressure zone at the catheter tip. This allows the catheter to break up and remove
thrombus. The waste tubing transports the thrombus debris from the catheter to the
collection bag for ultimate disposal.
INTENDED USE/INDICATIONS FOR USE
The Console is intended for use only in conjunction with an AngioJet Ultra
Thrombectomy Set. Refer to the individual Thrombectomy Set Directions for Use
manual for specific clinical applications.
CONTRAINDICATIONS
Refer to the individual Thrombectomy Set Directions for Use manual for specific
contraindications.
WARNINGS AND PRECAUTIONS
The AngioJet™ Ultra System should be used only by operators who have received
appropriate training on its installation and use.
• UsetheAngioJet™Ultra5000AConsoleonlywithanAngioJetUltra
Thrombectomy Set. This Console will not operate with a previous model pump set
and catheter.
• DonotattempttobypassanyoftheConsolesafetyfeatures.
• Ifthecatheterisremovedfromthepatientand/orisinoperative,thewaste
tubing lumen, guide catheter, and sheath should be flushed with sterile,
heparinized solution to avoid thrombus formation and maintain lumen patency.
Reprime the catheter by submerging the tip in sterile, heparinized solution and
operating it for at least 20 seconds before reintroduction to the patient.
• Physiciandiscretionwithregardtotheuseofheparinisadvised.
• RefertotheindividualAngioJetUltraThrombectomySetDirections for Use
manual for specific warnings and precautions.
• Donotmovethecollectionbagduringcatheteroperationasthismaycausea
collection bag error.
• Monitorthromboticdebris/fluidflowexitingthecatheterthroughthewaste
tubing during use. If blood is not visible during console activation, the catheter
may be occlusive within the vessel or the outflow lumen may be blocked.
• Ensureadequatepatientanticoagulationtopreventthrombusformationin
outflowlumen.
• RefertoindividualThrombectomySetDirections for Use manual for specific
instructions regarding heparinization of the Thrombectomy Set.
• TheConsolecontainsnouser-serviceableparts.Referserviceto
qualifiedpersonnel.
• Removalofoutercoversmayresultinelectricalshock.
• Thisdevicemaycauseelectromagneticinterferencewithotherdeviceswhen
in use. Do not place Console near sensitive equipment when operating.
• Equipmentnotsuitableforuseinthepresenceofflammableanestheticmixture
with air or with oxygen or nitrous oxide.
• Toavoidtheriskofelectricshock,thisequipmentmustonlybeconnectedtoa
supply mains with protective earth.
• Wherethe“Trapping Zone Hazard for Fingers” symbol is displayed on the
console, there exists a risk of trapping or pinching fingers during operation and
care must be exercised to avoid injury.
• Donotrepositionorpushtheconsolefromanypointotherthanthehandle
designed for that purpose. A condition of overbalance or tipping may ensue.
• TheAngioJetUltra5000AConsoleshouldnotbeusedadjacenttoorstacked
with other equipment, and if adjacent or stacked use is necessary, the AngioJet
Ultra 5000A Console should be observed to verify normal operation in the
configuration in which it will beused.
• Portableandmobileradiofrequency(RF)communicationsequipmentcanaffect
MEDICAL ELECTRICAL EQUIPMENT.
• Theuseofaccessoriesandcablesotherthanthosespecified,withthe
exception of accessories and cables sold by Boston Scientific as replacement
parts for internal components, may result in increased EMISSIONS or
decreased IMMUNITY of the Ultra 5000A Console.
• MEDICALELECTRICALEQUIPMENTneedsspecialprecautionsregarding
Electro-Magnetic Compatibility (EMC) and needs to be installed and put into
service according to the EMC information provided in the tables provided
below.
POTENTIAL ADVERSE EFFECTS
Refer to the individual Thrombectomy Set Directions for Use manual for specific
observed and/or potential adverse events.
HOW SUPPLIED
This product is supplied non-sterile and is intended for multiple use.
Do not use if package is opened or damaged.
Do not use if labeling is incomplete or illegible.
Handling and Storage
Operating Environment
Temperature: 10 °C to 40 °C (50 °F to 104 °F)
Relative Humidity: 30% to 75% (noncondensing)
Atmospheric Pressure: 700 hPa to 1060 hPa
Transport Environment
Temperature: -25 °C to 55 °C (-13 °F to 131 °F)
Relative Humidity: 10% to 95% (noncondensing)
Atmospheric Pressure: 500 hPa to 1060 hPa
Storage Environment
Temperature: 10 °C to 40 °C (50 °F to 104 °F)
Relative Humidity: 30% to 75% (noncondensing)
Atmospheric Pressure: 700 hPa to 1060 hPa
Pump
Catheter
Delivery and
waste tubing
Collection
Bag