Boston Scientific VERCISE DBS Owner's manual
Other Boston Scientific Medical Equipment manuals

Boston Scientific
Boston Scientific LATITUDE User manual

Boston Scientific
Boston Scientific Precision Spectra OMG SC-9315 User manual
Boston Scientific
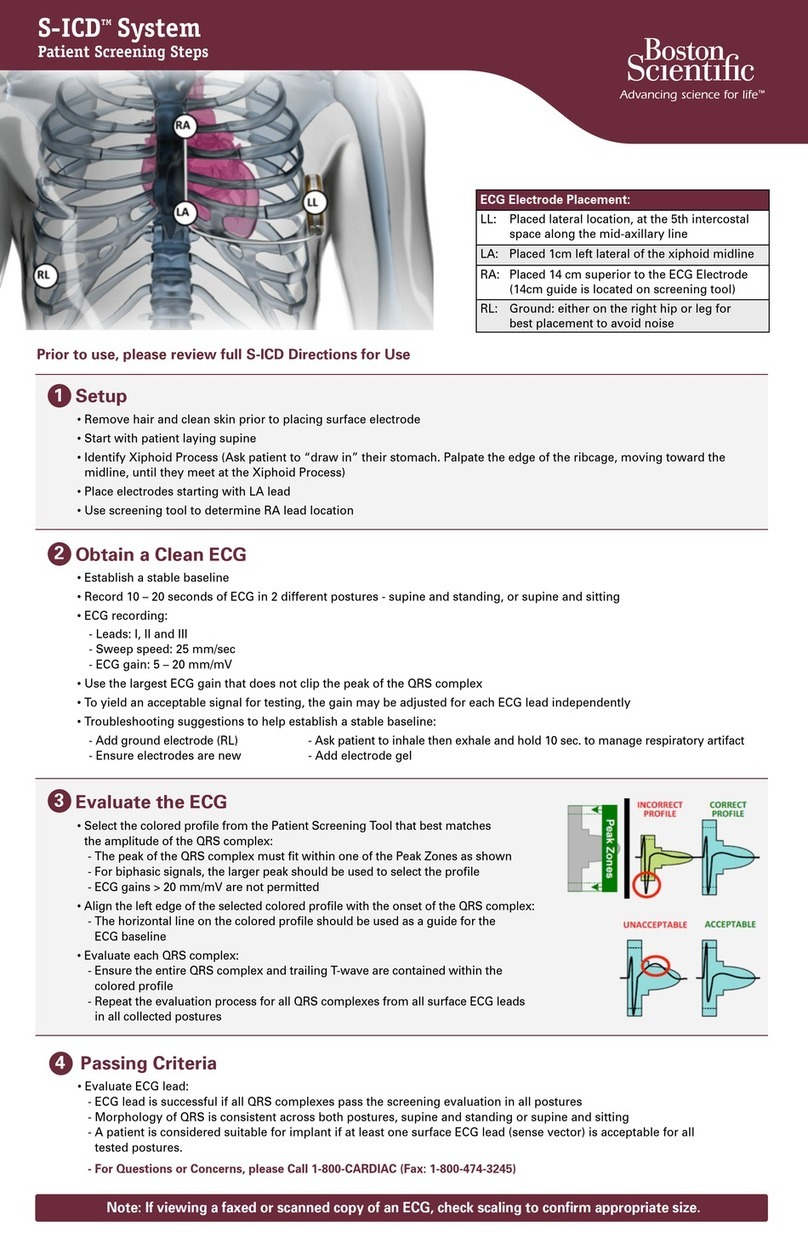
Boston Scientific S-ICD System Manual

Boston Scientific
Boston Scientific Precision Manual

Boston Scientific
Boston Scientific Precision SCS System Series Manual
Boston Scientific
Boston Scientific Precision SC-5312 User manual

Boston Scientific
Boston Scientific VERCISE DBS User manual

Boston Scientific
Boston Scientific Bionic Navigator 3d NM-7153-11A Owner's manual

Boston Scientific
Boston Scientific SC-2016 Series Manual
Boston Scientific
Boston Scientific VERCISE Series User manual

Boston Scientific
Boston Scientific Vercise DBS Charging User manual

Boston Scientific
Boston Scientific SC-5110 User manual

Boston Scientific
Boston Scientific Vercise PC Implantable Pulse Generator Manual

Boston Scientific
Boston Scientific precision spectra Manual
Boston Scientific
Boston Scientific Swiss LithoCast Trilogy FT-235 User manual

Boston Scientific
Boston Scientific Vercise Owner's manual

Boston Scientific
Boston Scientific EKOS Control System 4.0 User manual

Boston Scientific
Boston Scientific Rotablator Manual

Boston Scientific
Boston Scientific SpyGlass DS User manual

Boston Scientific
Boston Scientific Vercise PC Manual
Popular Medical Equipment manuals by other brands

Getinge
Getinge Arjohuntleigh Nimbus 3 Professional Instructions for use

Mettler Electronics
Mettler Electronics Sonicator 730 Maintenance manual

Pressalit Care
Pressalit Care R1100 Mounting instruction

Denas MS
Denas MS DENAS-T operating manual

bort medical
bort medical ActiveColor quick guide

AccuVein
AccuVein AV400 user manual

























