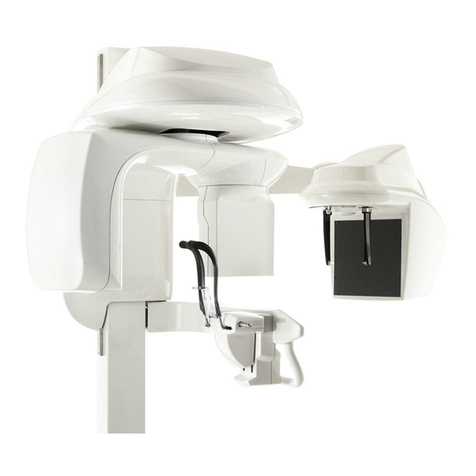
Quantum Medical Imaging, LLCQuantum Medical Imaging, LLC
CHAPTER 1 - SAFETY NOTICESCHAPTER 1 - SAFETY NOTICES
INFORMATION .........................INFORMATION .........................
..............................
..............................
NOTES .................NOTES .................
..............................
..............................
..............................
................................
..............................
..............................
CLASSIFICATION CLASSIFICATION
..............................
................................
................................
................................
COMPATIBILITY COMPATIBILITY
..............................
..............................
..............................
............................
..............................
............................
..............................
..............................
..............................
ACCOMPANYING DOCUMENTATION ....................ACCOMPANYING DOCUMENTATION ....................
............................
............................
APPLICABLE STANDARDS ...............APPLICABLE STANDARDS ...............
............................
..............................
..............................
DISPOSAL OF BATTERIES AND ACCUMULATORSDISPOSAL OF BATTERIES AND ACCUMULATORS
................................
..................................
................................
..........................
ELECTROMAGNETIC COMPATIBILITYELECTROMAGNETIC COMPATIBILITY
60601-1-2:2007/IEC 60601-1-2:2007/IEC
60601-1-2:2007) ......60601-1-2:2007) ......
................................
..................................
ABBREVIATION DEFINITION ABBREVIATION DEFINITION
..............................
..............................
..............................
..............................
CHAPTER 2 - GENERAL CHAPTER 2 - GENERAL
..............................
................................
..............................
..............................
..................................
..........................
..............................
..............................
..............................
..............................
SPECIFICATIONS SPECIFICATIONS
..........................
............................
..............................
..............................
..............................
............................
..............................
..............................
ACCESSORIES ACCESSORIES
..............................
..............................
..................................
..............................
..................................
............................
................................
..............................
..............................
CHAPTER 3 - OPERATIONCHAPTER 3 - OPERATION
..............................
................................
..............................
..............................
..................................
..............................
..............................
............................
............................
................................
..............................
..............................
............................
................................
..............................
..............................
Automatic Power Standby Mode ......................Automatic Power Standby Mode ......................
..............................
PROCEDURE ........................PROCEDURE ........................
..............................
EXPOSURES .................EXPOSURES .................
..................................
..................................
................................
............................
..............................
..............................
............................
..............................
..............................
............................
..............................
..............................
..............................
..............................
..............................
..............................
..........................
..............................
..............................
..............................
..............................