AF0118 | 2018-03-27 i
Contents
1Overview ............................................................................................................ 1-1
Preface ......................................................................................................................................... 1-1
About this Guide ......................................................................................................................... 1-1
Related Documentation .............................................................................................................. 1-2
Intended Audience ...................................................................................................................... 1-2
Indications for Use....................................................................................................................... 1-2
Usability ....................................................................................................................................... 1-2
Training........................................................................................................................................ 1-2
Safe Operation Precautions......................................................................................................... 1-3
Safety and Warning Symbols ...................................................................................................... 1-3
2Product Description ........................................................................................... 2-1
Features and Functions................................................................................................................ 2-1
3Operation and Use ............................................................................................. 3-1
Operation and Use ...................................................................................................................... 3-1
Cart............................................................................................................................................... 3-2
Cart Maneuverability and Positioning ........................................................................................ 3-4
Environmental Requirements and Infection Control ................................................................. 3-6
Acoustic Noise Emission............................................................................................................... 3-6
Product Classification per IEC 60601-1........................................................................................ 3-6
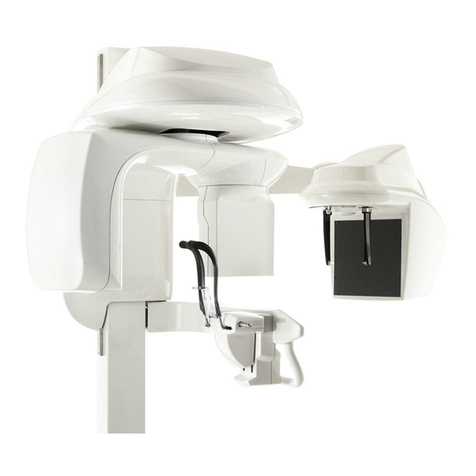
Arm and Tube Head Assembly .................................................................................................... 3-7
Exposure Factors and the X-ray Tube ....................................................................................... 3-13
Time Cycle between Exposures ................................................................................................. 3-15
Component Descriptions ........................................................................................................... 3-16
4Operating the System........................................................................................ 4-1
Drive the Cart............................................................................................................................... 4-1
Dock and Deploy the Arm........................................................................................................... 4-2
Operating the Machine ............................................................................................................... 4-2
Power Button Operation............................................................................................................. 4-3
5Maintenance and Safety Information .............................................................. 5-1
Cleaning Instructions................................................................................................................... 5-1
With Each Occurrence of Patient Contact .................................................................................. 5-1
Cleaning the Monitor .................................................................................................................. 5-2
Cleaning the Hardware ............................................................................................................... 5-3
Cleaning the Detector ................................................................................................................. 5-4
Cleaning the Plastic Components................................................................................................ 5-4
Cleaning the Arm ........................................................................................................................ 5-5
System Maintenance.................................................................................................................... 5-5