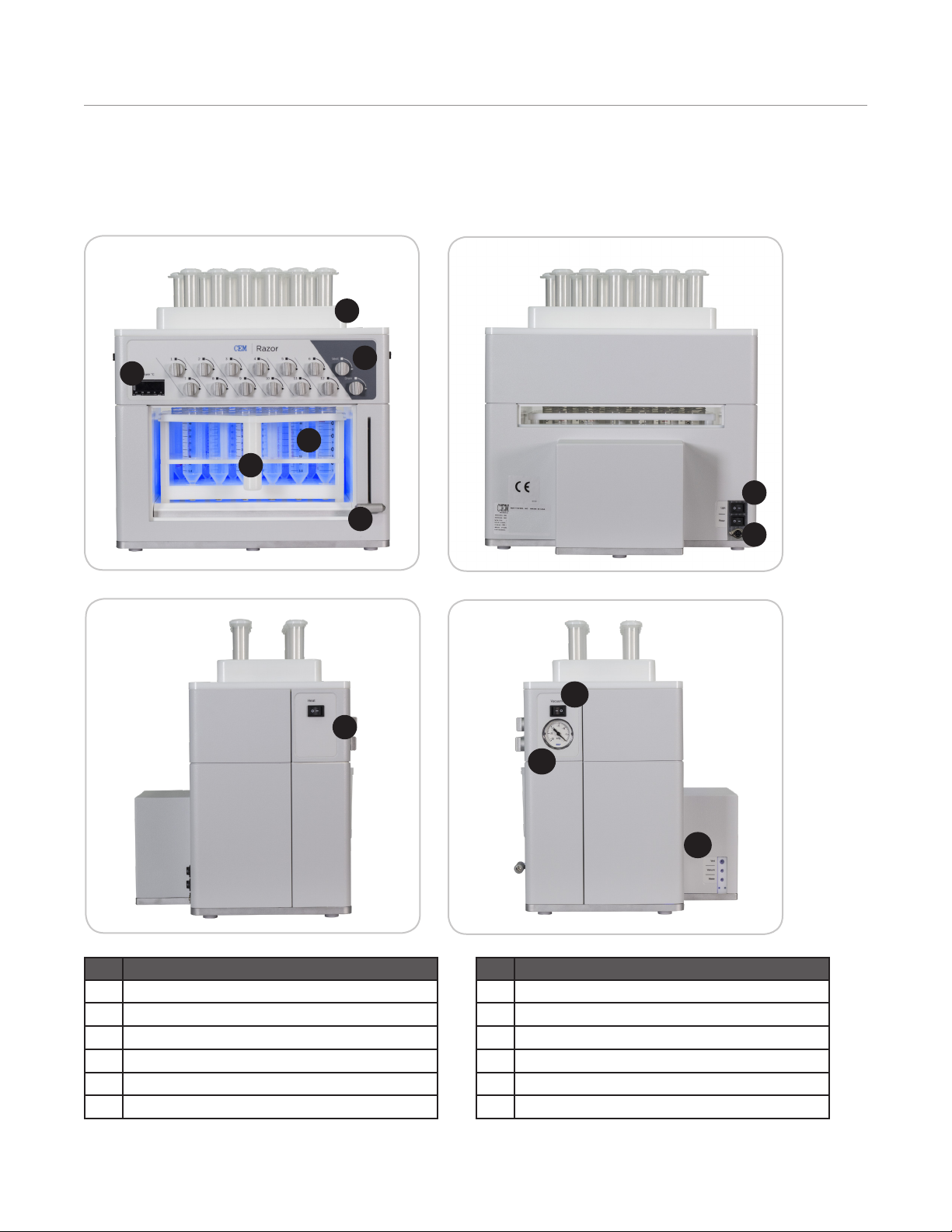
CEM Razor User manual
Other CEM Laboratory Equipment manuals

CEM
CEM Discover SP-D User manual

CEM
CEM Discover SP-D 10/35 Configuration guide

CEM
CEM liberty User manual

CEM
CEM DT-982 User manual

CEM
CEM MARS 6 User manual

CEM
CEM Discover SPS User manual

CEM
CEM MARS 6 User manual

CEM
CEM Q-Dry User manual

CEM
CEM Razor User manual

CEM
CEM Discover Protein Hydrolysis User manual

CEM
CEM EDGE User manual

CEM
CEM Smart Trac II User manual

CEM
CEM DT-986H User manual

CEM
CEM iPrep Series User manual

CEM
CEM MARS Series User manual

CEM
CEM EasyPrep Series User manual

CEM
CEM MARS 6 iWave User manual

CEM
CEM Liberty Blue 551290 User manual

CEM
CEM EDGE User manual

CEM
CEM MARSXpress Plus User manual
Popular Laboratory Equipment manuals by other brands

Belden
Belden HIRSCHMANN RPI-P1-4PoE installation manual

Koehler
Koehler K1223 Series Operation and instruction manual

Globe Scientific
Globe Scientific GCM-12 quick start guide

Getinge
Getinge 86 SERIES Technical manual

CORNING
CORNING Everon 6000 user manual

Biocomp
Biocomp GRADIENT MASTER 108 operating manual