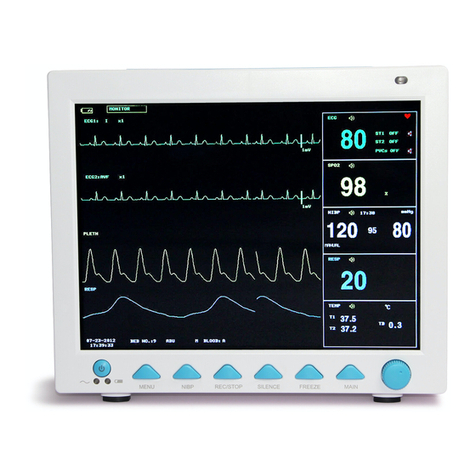
tOO
!l{l
85
1m
AV01'l1.llilt
Sa02
and
Sp02
Pules rate Bargraph Dispaly
•
Figure 3Bland-Altman plot
75
10
Figure
5.
''"'
8
...
Of;
I7
~
'a.
6
II'> Ii
((;
g4
'"
3
~
....
2
....
...
1
Q
~
N0
~
-]
I,W
-z
<;,
'3
l>!,;
~i'ii
'4
"
~J.
""5
0
-6
"'~
-1
...
5
·8
<It>
II
,"-----
Figure 4. Front View
•
Pules rate
---
......
~8
88
The display Sp02
------1++++88
~-t+t+!t------
Figure 2
SpOz
regression plot
~
One hanging rope;
~
Two batteries (optional);
~
One User Manual.
5
Accessories.
.
6Installation
6.1 View
of
the
Front
Panel
7
Operating
Guide .
6.2
Battery
Step
1.
Insert the two
AAA
size batteries properly in the right direction.
Step 2. Put the cover.
~lease
take
care
when
you
insert
the
batteries for
the
improper
insertion
may
damage
the
device.
6.3
Mounting
the
Hanging
Rope
Step
1.
Put the end
of
the rope through·the hole.
Step 2. Put another end
of
the rope through the first one and then tighten it.
4Technical Specifications
\
Glow and Infrared-ray
Receipt Tube
instrument is: Photoelectric Oxyhemoglobin Inspection Technology is adopted in accordance with Capacity Pulse Scanning &Recording
Technology~
so
that two beams
of
different wavelength
of
lights can be focused onto human nail tip through perspective clamp finger-type sensor. Then measured signal
can be obtained by aphotosensitive element, information acquired through which will be shown on screen through treatment in electronic circuits and
microprocessor.
7.4 Press the switch button once on front panel.
7.5 Do not shake the fmger and keep the user at ease during the process. Meanwhile, human body is not recommended in movement status.
7.6 Get the information directly from screen display.
7.7 In boot-strap state,press button ,and the device is reset.
~
Fingernails
and
the
luminescent
tube
should
be
on
the
same side.
Glow and Infrared-ray
Emission Tube
3.2
Caution
1.
The finger should be placed properly (see the attached illustration
of
this manual ,Figure 7), or else it may cause inaccurate measurement.
2. The
SpOz
sensor and photoelectric receiving tube should be arranged in away with the subject's arteriole in aposition there between.
3.
The SpOz sensor should not be used at alocation or limb tied with arterial canal or blood pressure cufforreceiving intravenous injection.
4.
Make sure the optical path
is
free from any optical obstacles like rubberized fabric.
5.
Excessive ambient light may affect the measuring result. It includes fluorescent
lamp~
dual ruby
light~
infrared heater, direct sunlight and etc.
6.
Strenuous action
of
the subject or extreme electrosurgical interference may also affect the accuracy.
7.
User can not use enamel or other makeup.
3.3 Clinical Restrictions
1.
As the measure is taken on the basis
of
arteriole pulse, substantial pulsating blood flow
of
subject is required. For asubject with weak pulse due to
shock, low ambientlbody temperature, major bleeding, or use
of
vascular contracting drug, the
SpOz
waveform (PLETH) will decrease. In this case,
the measurement will be more sensitive to interference.
2. For those with asubstantial amount
of
staining dilution drug (such as methylene blue, indigo green and acid indigo blue), or carbon monoxide
hemoglobin (COHb), or methionine (Me+Hb) or thiosalicylic hemoglobin, and some with icterus problem, the SpOz determination by this monitor
may be inaccurate.
3.
The drugs like dopamine, procaine, prilocaine, lidocaine and butacaine may also be amajor factor blamed for serious error
of
SpOz measure.
4. As the SpOz value serves as areference value for judgement
of
anemic anoxia and toxic
anoxia~
some users with serious anemia may also report
good SpOz measurement.
8Re airing
and
Maintenance
7.1
Insert the two batteries properly
to
the direction, and then put the cover.
7.2 Open the
clip.
7.3 Let the user's finger put into the rubber cushions
of
the clip (make sure the finger is in the right position), and
then!
clip the finger. As shown in
Figure 5
1)
Display
Format:
Digital tube Display;
SpOz
Measuring
Range: 0% -100%;
Pulse
Rate
Measuring
Range: 30 bpm -250 bpm;
Pulse Intensity Display: columniation display
2) Power Requirements: 2x1.5V
AAA
alkaline battery, adaptable range: 2.6V-3.6V.
3) Power
Consumption:
Smaller than
25
rnA.
4) Resolution: 1% for
SpOz
and 1bpm for Pulse Rate.
5)
Measurement
Accuracy: ±2% in stage
of
70%-100% SpOz, and meaningless when stage being smaller than 70%. ±2 bpm or±2% (select larger) for
Pulse Rate. Clinical Trial :SpOz regression plot &Bland-Altman plot,Refer to Figure 2&Figure
3.
6)
Measurement
Performance
in
Weak
Filling Condition: SpOz and pulse rate can be shown correctly when pulse-filling ratio is 0.4%.
SpOz
error
is
±4%, pulse rate error is ±2 bpm or ±2% (select larger).
7) Resistance to
surrounding
light: The deviation between the value measured in the condition
of
man-made light, indoor natural light and that
of
darkroom
is
less than
±l
%.
8)
It
is
equipped with aswitch function. The Oxirpeter can be powered offwhen the finger
is
off
the oximeter within 5seconds.
9) Optical Sensor
Red light (wavelength is 660nm, 6.65mW)
Infrared (wavelength is 880nm, 6.75mW)
Figure
1.
Operating Principle
~
Please change the batteries when the low-voltage displayed on the screen.
~
Please clean the surface
of
the device before using. Wipe the device with medical alcohol first, and then let it dry in air or clean it by dry clean fabric.
~
Using the medical alcohol to disinfect the product after use, prevent from cross infection for next time use.
~
Please take out the batteries
if
the oximeter is not in use for along time.
~
The packed device can be transported by ordinary conveyance or according to transport contract.The device can not be transported mixed with toxic,
harmful, corrosive material.
~
The best storage environment
of
the device is -40°C to
60°C
ambient temperature and not higher than 95% relative humidity.
~
Users are advised to calibrate the device termly (or according to the calibrating program
of
hospital). It also can be performed at the state-appointed
agent orjust contact us for calibration.
&.
High-pressure sterilization
cannot
be
used on
the
device.
&.
Do
not
immerse
the
device
in
liquid.
&.
It
is recommended
that
the
device should
be
kept
in a
dry
environment. Humidity
may
reduce
the
useful life
of
the
device,
or
even
damage
File No.:CMS2.782.395(OCH)ESS/1.0
Release Date: June 2016
1.4.01.01.393
Address: No.112 Qinhuang West Street, Economic &Technical DevelopmentZone,
Qinhuangdao, Hebei Province, PEOPLE'S REPUBLIC OF CHINA
Tel:
0086-335-8015430
Fax: 0086-335-8015588
Technical support: 0086-335-8015431
Website: http://www.contecmed.com
CONTEC™
Contec
Medical
Systems
Co.,
Ltd.
Instructions to User
recommended to
be
used
under
this circumstance.
The pulse oxygen saturation is the percentage
of
HbOz in the total Hb in the blood, so-called the
Oz
concentration in the blood. It is an important
bio-parameter for the respiration. For the purpose
of
measuring the
SpOz
more easily and accurately, our company developed the Pulse Oximeter. At the
same time, the device can measure the pulse rate simultaneously.
The Pulse Oximeter features in small volume, low power consumption, convenient operation and being portable. It is only necessary for user to put one
of
his fmgers into afingertip photoelectric sensor for diagnosis, and adisplay screen will directly show measured value
of
Hemoglobin Saturation.
2.1 Features
~
Operation
of
the product is simple and convenient.
~
The product is small in volume, light in weight (total weight is about 50g including batteries) and convenient in carrying.
~
Power consumption
of
the product is low and the two originally equipped AAAbatteries can be operated continuously for 24 hours.
~
The product will automatically be powered
off
when no signal is in the product within 5seconds.
~
Low -battery indicator as battery icon flash manner.
2.2
Major
Applications
and
Scope
of
Application
The Pulse Oximeter can be used in measuring pulse oxygen saturation and pulse rate through finger. The product is suitable for family use(It can be used
before or after doing sports, and it is not recommended to use the device during the process
of
doing sports) .
~
The
problem
of
overrating would emerge when
the
user
is suffering from toxicosis which caused
by
carbon
monoxide,
the
device is
not
CMS50M
2Overview
Dear users, thank you very much for purchasing the Pulse Oximeter.
In case
of
modifications and software upgrades, the information contained in this document is subject to change without notice.
The Manual describes, in accordance with the Pulse Oximeter's features and requirements, main structure, functions, specifications, correct methods for
transportation, installation, usage, operation, repair, maintenance and storage, etc. as well as the safety procedures to protect both the user and equipment.
Refer to the respective chapters for details.
Please read the User Manual carefully before using this product. The User Manual which describes the operating procedures should be followed
strictly.Failure to follow the User Manual may cause measuring abnormality, equipment damage and human injury. The manufacturer is NOT responsible
for the safety, reliability and performance issues and any monitoring abnormality, human injury and equipment damage due to users' negligence
of
the
operation instructions. The manufacturer's warranty service does not cover such faults.
Owing to the forthcoming renovation, the specific products you received may not be totally in accordance with the description
of
this User Manual.
We
would sincerely regret for that.
This product can be used repeatedly. The operating life is 3years.
If
you have any questions regarding to the use
of
this product, please call
us
at 1-847-562-1702 Monday-Friday from 8:00 AM to 5:00 PM Eastern Time.
WARNING:
~
Uncomfortable
or
painful feeling may
appear
if
using
the
device
cea~elessly,
especially for
the
the
microcirculation
barrier
users.
It
is
recommended
that
the
sensor should
not
be
applied to
the
same finger for
over
2hours.
r
For
the
special users,
there
should
be
a
more
prudent
inspecting in
the
placing process.
The
device
can
not
be
clipped on
the
edema
and
tender
tissue.
~
The
light (the
infrared
is invisible)
emitted
from
the
device is
harmful
to
the
eyes, so
the
user
and
the
maintenance
man
should
not
stare
at
the
light.
rUser
can
not
use enamel
or
other
makeup.
r
User's
fingernail can
not
be
too long.
rPlease refer to
the
correlative
literature
about
the
clinical restrictions
and
caution.
rThis device is
not
intended
for
treatment.
The User Manual
is
published by our company. All rights reserved.
Pulse
Oximeter
(UJ~~lr
[tMtl~lJillYl~O
1Safety
1.1 Instructions for Safe
Operations
~
Check the main unit and all accessories periodically to make sure that there is no visible damage that may affect user's safety and monitoring
performance about cables and transducers. It is recommended that the device should be inspected once aweek at least. When there is obvious
damage, stop using the oximeter .
~
Necessary maintenance must be performed by qualified engineers
ONLY.
Users are not permitted to maintain it by themselves.
~
The oximeter cannot be used together with devices not specified
in
User's Manual.Only the accessory that appointed or recommendatory by
manufacture can be used with this device.
~
This product is calibrated before leaving factory.
1.2 Warnings
~
Explosive
hazard-DO
NOT use the oximeter in environment with inflammable gas such
as
some ignitable anesthetic agents.
~
The person who is allergic to rubber can not use this device.
~
The disposal
of
scrap instrument and its accessories and packings(including battery, plastic bags, foams and paper boxes) should follow the local
laws and regulations.
~
Please check the packing before use to make sure the device and accessories are totally in accordance with the packing list, or else the device may
have the possibility
of
working abnormally.
~
Please don't measure this device with function test paper for the device's related information.
~
Parts
of
the device that are not serviced or maintained while in use with the user.
};-
WarninKagainst servicing and maintenance while the me equipment is
in
use.
~
No modification
of
this equipment is allowed.
~
The user
is
an intended operator.
~
The probe
of
the device is the applied part.
1.3 Attentions
GKeep the oximeter away from dust, vibration, corrosive substances, explosive materials, high temperature and moisture.
G
If
the oximeter gets wet, please stop operating it.
GWhen it
is
carried from cold environment to warm or humid environment, please do not use it immediately.
GDO NOT operate keys on front panel with sharp materials.
GHigh temperature or high pressure steam disinfection
of
the oximeter is not permitted. Refer to User Manual in the relative chapter for instructions
of
cleaning and disinfection.
GDo not have the oximeter immerged in liquid. When it needs cleaning, please wipe its surface with medical alcohol by soft material. Do not spray
any liquid on the device directly.
GWhen cleaning the device with water, the temperature should be lower than 60°C.
GAs to the fingers which are too thin or too cold, it would probably affect the normal measure
of
the users'
Sp02
and pulse rate, please clip the thick
finger such as thumb and middle finger deeply enough into the probe.
GDo not use the device on infant or neonatal users.
GThe product
is
suitable for adults(Weight should be between 40kg to 1IOkg).
GThe device may not work for all users.
If
you are unable to achieve stable readings, discontinue use.
GThe update period
of
data is less than 5seconds, which
is
changeable according to different individual pulse rate.
G
If
some abnormal conditions appear on the screen during test process, pull out the finger and reinsert to restore normal use.
GThe hanging rope attached the product is made from Non- allergy material,
if
particular group are sensitive to the hanging rope, stop using it. In
addition, pay attention to the use
of
the hanging
rope,
do not wear it around the neck avoiding cause harm to the users.
GThe instrument dose not have low-voltage alarm function, it only shows the low-voltage,please change the battery when the battery energy is used
out.
GWhen the parameter
is
particularly, The instrument dose not have alarm function.Do not use the device in situations where alarms are required.
GBatteries must be removed
if
the device is going to be stored for more than one month, orelse batteries may leak.
GAflexible circuit connects the two parts
of
the device. Do not twist or pull on the connection.
1.4.Indication for Use
The Pulse Oximeter is anon-invasive device intended for the spot-check
of
saturation
of
arterial hemoglobin(Sp02) and the pulse rate
of
adult in home
use environments.This device is not intended for continuous monitoring.The device can be multi-used.Solely for use with sporting and aviation
activities.Intended to monitor heart rate during exercise.
2.3
Environment
Requirements
Storage Environment
a) Temperature
:-40°C~+60°C
b) Relative humidity
::::s
95%
c) Atmospheric pressure
:500hPa~1060hPa
Operating Environment
a) Temperature:
:10°C~40°C
b) Relative Humidity
::::S75%
c) Atmospheric
pressure:700hPa~
1060hPa
3Principle
and
Caution it.
3.1 Principle
of
Measurement
Principle
of
the Oximeter is
as
follows: An experience formula
of
data process is established taking use
of
Lambert Beer Law according to Spectrum
Absorption Characteristics
of
Reductive Hemoglobin (Hb) and Oxyhemoglobin (HbOz) in glow &near-infrared zones. Operation principle
of
the