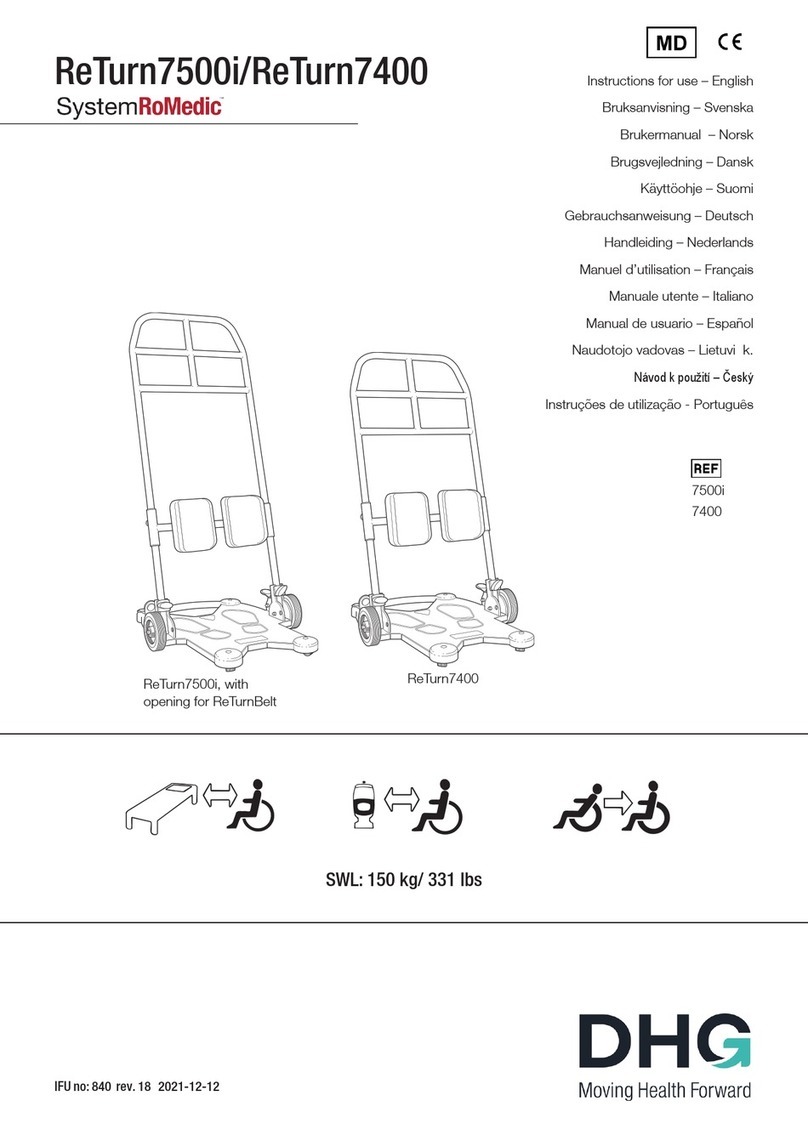
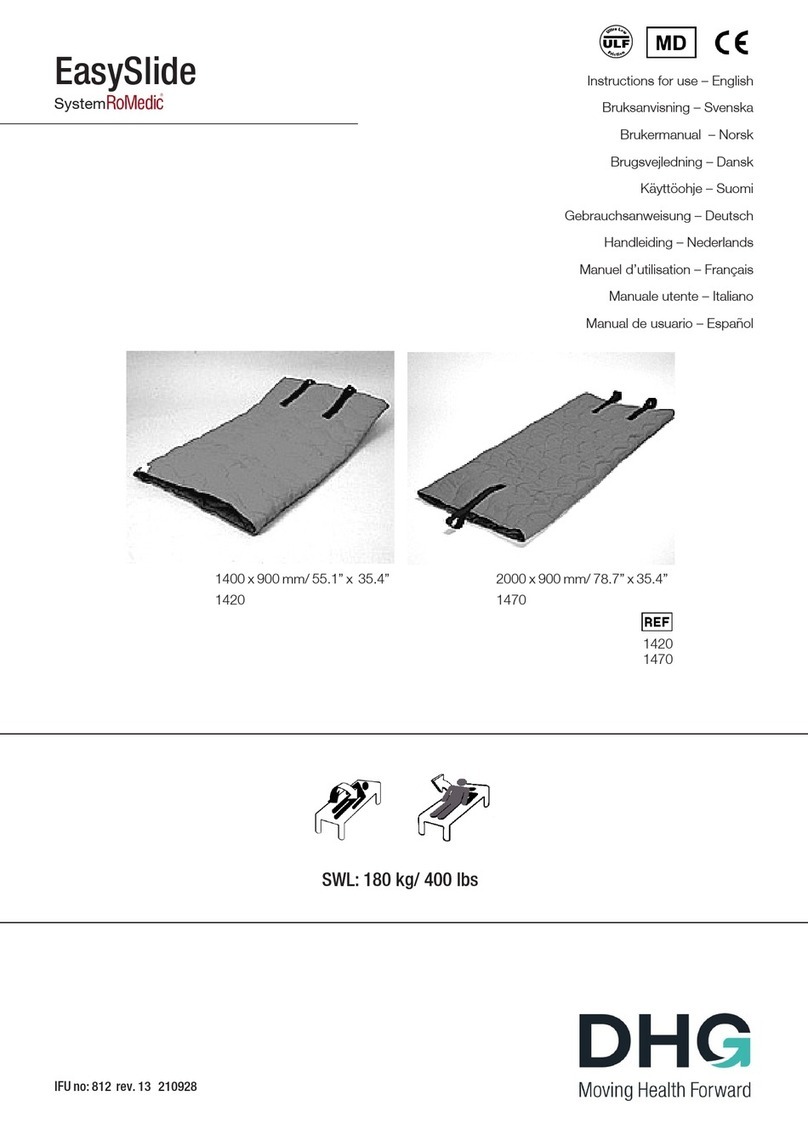
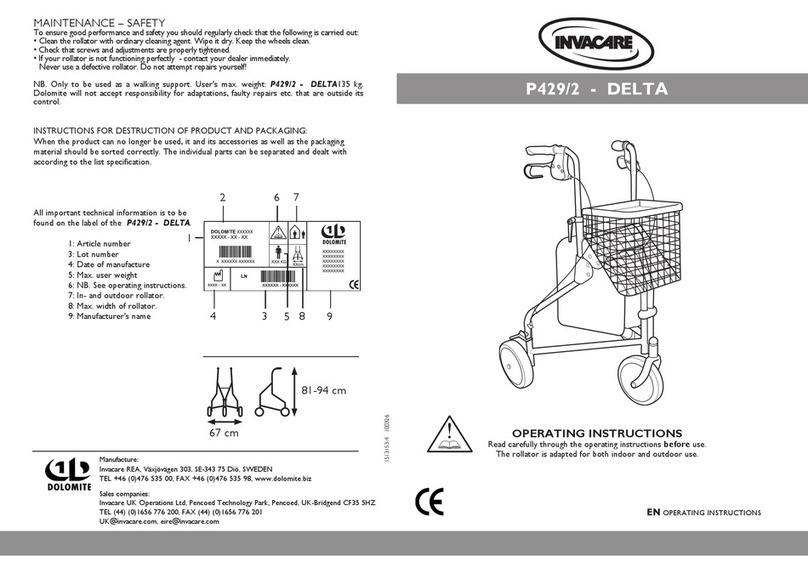
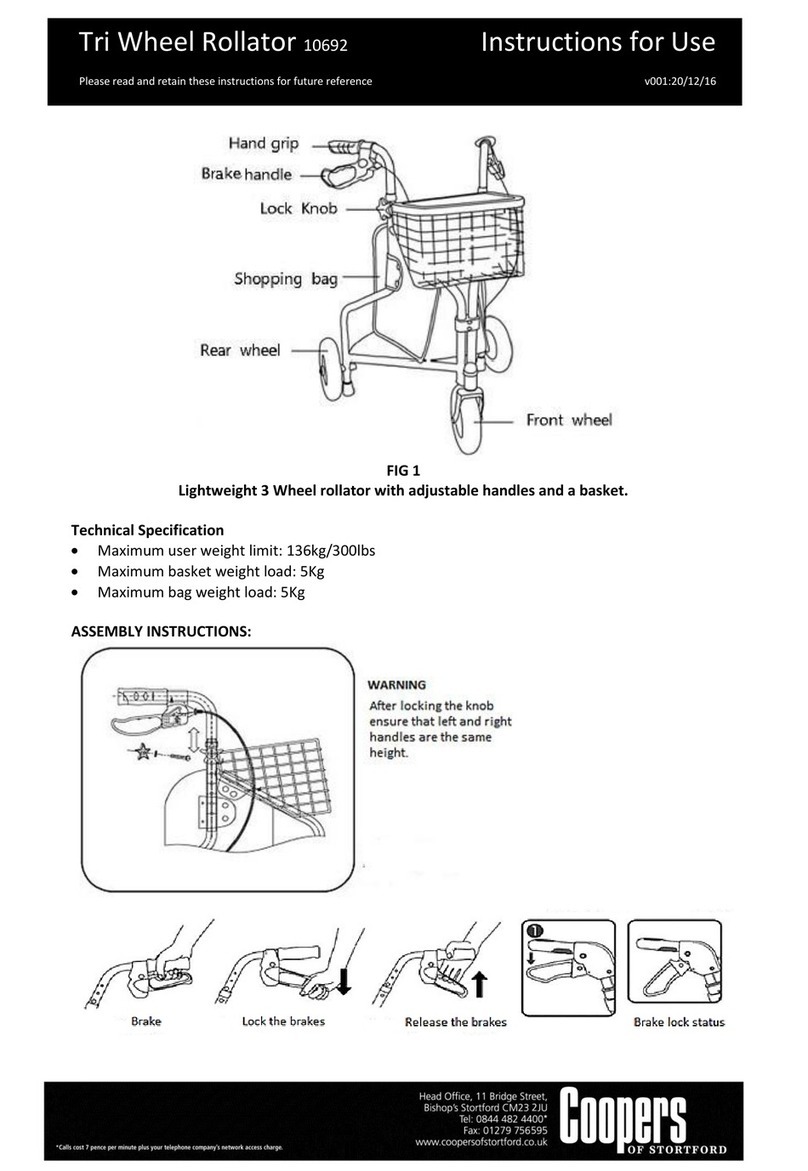
11
IFU
5. Maintenance
The device must undergo thorough inspection at least once per year. Inspection must be performed by authorised
personnel and in accordance with Direct Healthcare Group’s instructions.
Repairs and maintenance may only be done by authorised personnel using original spare parts.
Check that Action if a problem is noticed
General
The Walker feels firm/free from play.
The Walker does not rattle during manoeuvres.
The Walker is level and all of its castors are in
contact with the floor.
The Walker is not dirty.
Clean with lukewarm soap solution or alcohol-based cleaning
agent (no petroleum products).
Armrests
Armrests are intact and clean.
Armrest widening is functional. Clean; replace if damaged.
Replace lock knob.
Fit lock screws/plates as required.
Handle
Grips are not damaged/dirty.
Handle adjustment is functional. Clean with lukewarm soap solution (no petroleum products).
Replace handle grips.
Fit lock screws/plates as required.
Electronics
Check that the charging cable is connected to
the control box.
Check that the hand control is connected to
the control box.
Check that the battery, actuators and control
box are not loose.
If any part needs to be replaced refer to the wiring diagram in
the control box wiring section.
Fit new charging cable; this must always be connected to the
control box.
Connect or fit new hand control as required.
Tighten; replace with new fasteners as required.
Height adjustment
Raising and lowering are functional.
The Walker feels stable at maximum settings.
There is no play between the vertical frame
and bottom frame.
Height adjustment clamps lock.
The electric actuator fitting must be free from
play.
Height adjustment via hand control is
functional. The electric actuator must run
smoothly at a constant speed.
Fit new guide sleeves (in frame) or end plugs (in chrome
tubes) as required.
Tighten all lock bolts.
Replace clamps.
Tighten the attachment fitting concerned; replace bolts/lock
nuts as necessary.
Check that the battery/hand control/ actuator are connected in
accordance as in the control box wiring section.
Charge the battery.
The part list shows the design and which fasteners must be
checked.
Frame parts
There is no mechanical damage.
There are no scratches.
End plugs/lower frame fitted
If mechanical damage is present, contact DHG Customer
Services.
Touch up as necessary.
Fit new end plugs.
Castors/brakes
Castors roll easily/tread not damaged.
The castors are firmly fastened to the lower
frame.
Castor brakes functional on all castors.
Handbrake functional.
Clean or replace castors.
NOTE! Castors are always fixed to the lower frame with thread
locker or lock nuts. The castors are sealed and we do not
recommend their dismantling; instead, replace the whole
castor assembly.
Tighten the castor bolt and fit locking nut or use thread locker
(depends on model).
Replace castor assembly.
Adjust the brake or fit new castors.
Power cord may only be replaced by DHG service personnel or technical personnel trained by DHG.
DHG can provide upon request neccessary technical information in order to assist maintenace and repair of the
device.