TECHNICAL DATA
Specications:
Main Unit:
Dimensions: 2.6”X 5.2”(66 mm X 131 mm)
Weight: Approx. 0.5 lb (0.227 kg)
Mode of Operation: Cyclic
Source of Power: 7.4 volt Li-ion battery pack (made up of 2 – 3.7 volt cells)
CAUTION: Charge batteries using only the power source provided by
InnovaMed health.
USER MAINTENANCE
Contains no serviceable parts. Contact InnovaMed Health Customer Service at
877-475-7050
Inspect the unit and all components for any damage that may have occurred during shipping or general
handling prior to each use (for example, frayed or cut charging cord, cracked plastic housings, torn cus,
etc). Refer to image ofVenaPro for description of all components.
Do not attempt to connect the wall supply if any damage is noticed.
Avoid subjecting the units to shocks, such as dropping the pumps.
Do not handle the leg cus with any sharp objects. If a bladder is punctured or you notice a leak, do not
attempt to repair the unit or cus. Replacement units are available through customer service.
Avoid folding or creasing the bladder during use and transportation of the units.
Battery is not replaceable, replacement units are available through customer service.
Contact InnovaMed Health to receive replacements instructions for any damaged items.
STORAGE
Store in a dry location between +10⁰C (50⁰F) and +40⁰C (104⁰F).
Do not expose to heat exceeding 65⁰C (149⁰F).
Do not store items in direct sunlight.
DISPOSAL
This unit is an electromechanical device that includes printed circuit boards and rechargeable batteries.
Do not discard in landll. Consult local county requirements for proper disposal instructions.
Pump control units contain rechargeable batteries. Do not discard the pump unit in regular waste. Bring
the unit to your local recycle center or contact InnovaMed Health.
The use of accessories, power supplies and cables other than those specied, with the
exception of components sold by the manufacturer of theVenaPro as replacement
parts, may result in increased emissions or decreased immunity of the VenaPro.
Class II medical electrical equipment
This unit is a electromechanical device that includes printed circuit boards and
rechargeable batteries. Do not discard in landll. Consult local county requirements
for proper disposal instructions.
This symbol designates the degree of protection against electrical shock from the wrap
as being a type B applied part.
Follow the instructions for use.
This device is not protected against water. Equipment is not suitable for use in the presence of ammable
anesthetic mixture with air, oxygen, or nitrous oxide. The rechargeable batteries supplied in this unit
are not eld replaceable. If you have any issues please contact 877-475-7050 for a replacement unit.
POWER SUPPLY:
Class II, input: 100 - 240 Vac, 50 - 60 Hz, output: 10 Vdc @ 1.1 Amp)
Use only UL/60601-1 approved power supplies from InnovaMed Health for use
in hospital settings.
Output:
Mode of Operation: Continuous
SYSTEM OPERATING ENVIRONMENT:
Temperature: +10 Degrees C (50 Degrees F) to +40 degrees C (104 degrees F)
Humidity: 30%-75%
DEFAULT SETTINGS:
Leg Pressure (not adjustable) 50mmhg
Cycle time: 60 Seconds
TOLERANCES:
Pressure 5%
BATTERY CHARGE:
Takes approximately 6 hours (from
depleted state).
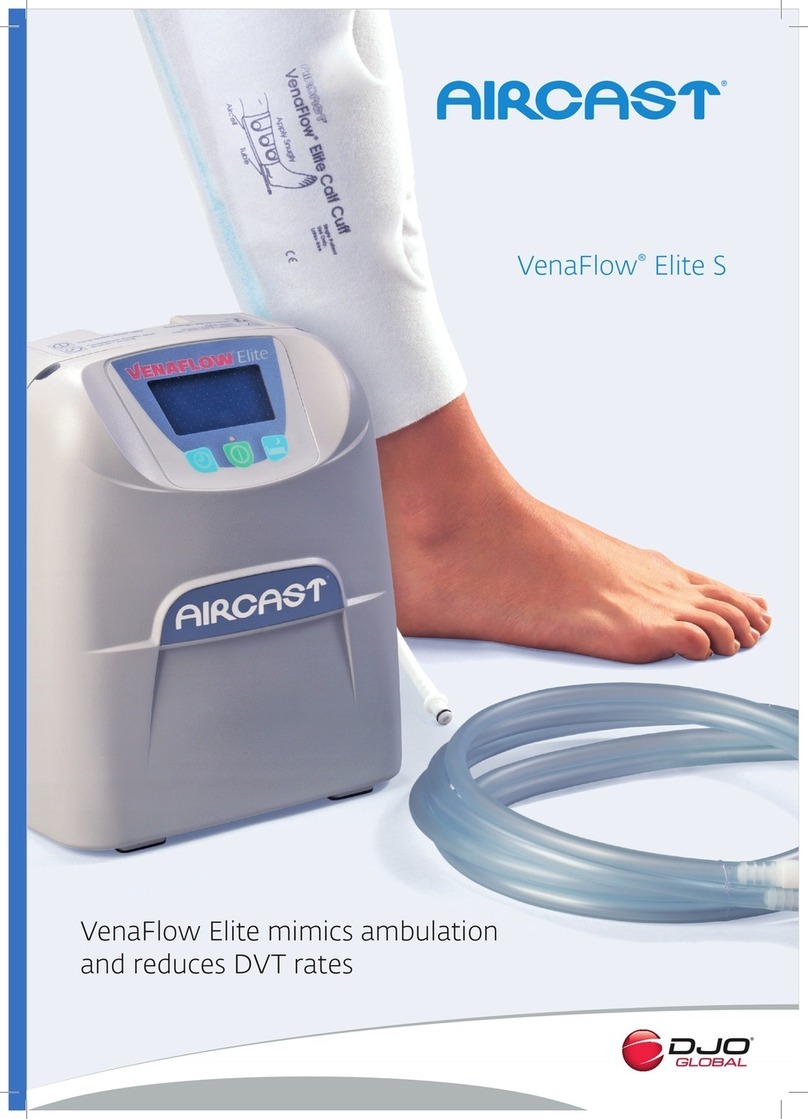
PURPOSE OF THIS DEVICE
The purpose of theVenaPro is to aid in the prevention of Deep Vein
Thrombosis (DVT) by helping to stimulate blood ow in the legs. This
is accomplished by an electronically controlled pump delivering a set
amount of air to the leg cus that, in turn, compress the calf or calves
to aid blood ow out of the lower extremities.
The pump will inate each leg cu to a preset pressure of 50mmhg and
deate once the pressure is reached.The cycles are repeated on each
unit until the power is turned o. Internal rechargeable batteries allow
theVenaPro to be completely portable, thus preventing interruptions
in treatment.
Indications for Use:
TheVena Pro Vascular Therapy System, model VP-3111, is intended to
be an easy to use portable system, prescribed by a physician, for use in
the home or clinical setting to help prevent the onset of DVT in patients
by stimulating blood ow in the extremities (simulating muscle
contractions).
This device can be used to:
Aid in the prevention of DVT
Enhance blood circulation
Diminish post-operative pain and swelling
Reduce wound healing time
Aid in the treatment of: stasis dermatitis, venous stasis ulcers, arterial
and diabetic leg ulcers, chronic venous insuciency and reduction of
edema in the lower limbs
As prophylaxis for DeepVein Thrombosis (DVT) by persons expecting to
be stationary for long periods of time
CONTRAINDICATIONS
TheVenaPro MUST NOT be used to treat the following conditions:
Persons with suspected, active or untreated: deep vein thrombosis,
ischemic vascular disease, severe arteriosclerosis, pulmonary edema,
severe congestive heart failure, thrombphlebitis, or an active infection.
On the legs where cus would interfere with the following conditions:
vein ligation, gangrene, dermatitis, open wounds, a recent skin graft,
massive edema or extreme deformity of the leg.
On any neuropathy.
On extremities that are insensitive to pain.
Where increased venous or lymphatic return is undesirable.