Esco Medical AVT-1 User manual

Rev. 1.8
Rx only
Anti-vibration Table (AVT-1)®

AVT-1® User Manual Rev. 1.8
2
For Technical Service, contact
North America
Esco Technologies, Inc.
903 Sheehy Drive, Suite F, Horsham, PA 19044, USA
Tel 215-441-9661 •Fax 484-698-7757
www.escolifesciences.us •eti.admin@escoglobal.com
Rest of the World
Esco Micro Pte. Ltd.
21 Changi South Street 1 •Singapore 486 777
Tel +65 6542 0833 •Fax +65 6542 6920
Copyright Information
© Copyright 2014 Esco Micro Pte Ltd. All rights reserved.
The information contained in this manual and the accompanying product is copyrighted and all
rights are reserved by Esco.
Esco reserves the right to make periodic minor design changes without obligation to notify any
person or entity of such change.
Caution: Federal law restricts this device to sale by or on the order of a licensed healthcare
practitioner.
Only to be used by a trained and qualified professional. The device is sold under exemption 21
CFR 801 Subpart D.
“Material in this manual is provided for informational purposes only. The contents and the
product described in this manual (including any appendix, addendum, attachment or inclusion)
are subject to change without notice. Esco makes no representations or warranties as to the
accuracy of the information contained in this manual. In no event shall Esco be held liable for
any damages, direct or consequential, arising out of or related to the use of this manual.”

AVT-1® User Manual Rev. 1.8
3
Unpacking and Inspection
Follow standard receiving practices upon receipt of the instrument. Check the ship-
ping carton for damage. If damage is found, stop unpacking the instrument. Notify
the freight carrier and ask for an agent to be present while the instrument is un-
packed. There are no special unpacking instructions, but be careful not to damage
the instrument when unpacking it. Inspect the instrument for physical damage such
as bent or broken parts, dents or scratches.
Claims
Our routine method of shipment is via a common carrier. Upon delivery, if physical
damage is found, retain all packing materials in their original condition and contact
the carrier immediately to file a claim.
If the instrument is delivered in a good physical condition but does not operate within
specifications, or if there are any other problems caused not during the shipping,
please contact your local sales representative or Esco Medical immediately.
Standard Terms and Conditions
Refunds & Credits
Please note that only the serialized products (products labelled with a distinct serial
number) and accessories are eligible for partial refund and/or credit. Non-serialized
parts and accessory items (cables, carrying cases, auxiliary modules, etc.) are not
eligible for return or refund. In order to receive a partial refund/credit, the product
must be undamaged and returned complete (meaning together with all manuals, ca-
bles, accessories, etc.) within 30 days of original purchase, in “as new” and resalable
condition. The Return Procedure must be followed.
Return Procedure
Every product returned for refund/credit must be accompanied by a Return Material
Authorization (RMA) number, obtained from Esco Medical Customer Service. All
items being returned must be sent prepaid (freight, duty, brokerage and taxes) to our
factory location.
Restocking Charges
Products returned within 30 days of original purchase are subject to a minimum re-
stocking fee of 20% of the list price. Additional charges for damage and/or missing
parts and accessories will be applied to all returns. Products which are not in “as
new” and resalable condition are not eligible for credit return and will be returned to
the customer at their own expense.
Certification

AVT-1® User Manual Rev. 1.8
4
This instrument had been thoroughly tested/inspected and found to meet Esco Medi-
cal´s manufacturing specifications when shipped from the factory. Calibration meas-
urements and testing are traceable and done according to Esco Medical ISO certifi-
cation.
Warranty and Product Support
Esco Medical warrants this instrument to be free from defects in materials and
workmanship under normal use, and service for two (2) years from the date of origi-
nal purchase, provided the instrument is calibrated and maintained in accordance
with this manual. During the warranty period Esco Medical will, at our option, either
repair or replace a product that proves to be defective at no charge, provided you
return the product (shipping, duty, brokerage and taxes prepaid) to Esco Medical.
Any and all transportation charges incurred are the responsibility of the purchaser
and are not included within this warranty. This warranty extends only to the original
purchaser and does not cover damage from abuse, neglect, accident or misuse, or
as the result of service or modification by parties other than Esco Medical.
IN NO EVENT SHALL ESCO MEDICAL LTD. BE HELD LIABLE FOR CONSE-
QUENTIAL DAMAGES.
No warranty shall apply when damage is caused by any of the following:
Power failure, surges or spikes
Damage in transit or when moving the instrument
Improper power supply such as low voltage, incorrect voltage, defective wiring or
inadequate fuses
Accident, alteration, abuse or misuse of the instrument
Fire, water damage, theft, war, riot, hostility, force majeure such as hurricanes,
floods, etc.
Only serialized products (those items bearing a distinct serial number tag) and their
accessory items are covered under this warranty.
PHYSICAL DAMAGE CAUSED BY MISUSE OR PHYSICAL ABUSE IS NOT COV-
ERED UNDER THE WARRANTY. Items such as cables and non-serialized modules
are not covered under this warranty.
This warranty gives you specific legal rights and you may have other rights, which
vary from province to province, state to state or country to country. This warranty is
limited to repairing the instrument per Esco Medical's specifications.
When you return an instrument to Esco Medical for service, repair or calibration, we
recommend shipping the instrument in the original shipping foam and container. If
the original packing materials are not available, we recommend the following guide
for repackaging:

AVT-1® User Manual Rev. 1.8
5
Use a double-walled carton of sufficient strength for the weight being shipped
Use heavy paper or cardboard to protect all instrument surfaces. Use non-abrasive
material around all projecting parts
Use at least four inches of tightly packed, industrial-approved, shock-absorbent
material all around the instrument
Esco Medical will not be held responsible for lost shipments or instruments received
in damaged condition due to improper packaging or handling. All warranty claim
shipments must be made on a prepaid basis (freight, duty, brokerage, and taxes). No
returns will be accepted without a Return Materials Authorization ("RMA”) number.
Please contact Esco Medical to obtain an RMA number and receive help with
shipping/customs documentation.
Recalibration of instruments, which to be calibrated according to the recommended
annual calibration frequency, is not covered by the warranty.
Warranty Disclaimer
Should you choose to have your instrument serviced and/or calibrated by someone
other than Esco Medical Ltd. and their representatives, please be advised that the
original warranty covering your product becomes void when the tamper-resistant
Quality Seal is removed or broken without proper factory authorization.
In all cases, breaking the tamper-resistant Quality Seal should be avoided at all
costs, as this seal is key to your original instrument warranty. In an event where the
seal must be broken to gain internal access to the instrument, you must first contact
Esco Medical Ltd.
You will be required to provide us with the serial number for your instrument, as well
as a valid reason for breaking the Quality Seal. You should break this seal only after
you receive factory authorization. Do not break the Quality Seal until you have con-
tacted us! Following these steps will help ensure that you will retain the original war-
ranty on your instrument without interruption.
WARNING
Unauthorized user modifications or application beyond the published specifications
may result in electrical shock hazard or improper operation. Esco Medical will not be
held responsible for any injury sustained due to unauthorized equipment modifica-
tions.
ESCO MEDICAL LTD. DISCLAIMS ALL OTHER WARRANTIES, EXPRESSED OR
IMPLIED, INCLUDING ANY WARRANTY OF MERCHANTABILITY OR FITNESS
FOR A PARTICULAR PURPOSE OR APPLICATION.
THIS PRODUCT DOES NOT CONTAIN ANY USER-SERVICEABLE

AVT-1® User Manual Rev. 1.8
6
COMPONENTS.
UNAUTHORIZED REMOVAL OF THE INSTRUMENT COVER SHALL VOID THIS
AND ANY OTHER EXPRESSED OR IMPLIED WARRANTIES.

AVT-1® User Manual Rev. 1.8
7
Table of contents
1 How to use this manual...........................................................................................8
2 Safety warning ........................................................................................................8
3 About the product....................................................................................................8
4 Accessories supplied...............................................................................................9
5 Safety symbols and labels.......................................................................................9
6 Important safety instructions and warnings...........................................................10
6.1 Before installation...........................................................................................10
6.2 During installation...........................................................................................10
6.3 Post installation...............................................................................................10
7 Getting started.......................................................................................................10
8 Cleaning instructions.............................................................................................13
9 Maintenance..........................................................................................................14
10 User troubleshooting...........................................................................................14
11 Specifications......................................................................................................15

AVT-1® User Manual Rev. 1.8
8
1 How to use this manual
The manual is designed to be read by sections and not ideally from cover to cover. This
means that if the manual is read from start to finish, there will be some repetition and
overlap. We recommend the following method for going through the manual: first, famil-
iarize yourself with the safety instructions; then proceed to the basic user functions that
are needed for operating the equipment on a day to day basis; then review the alarm
functions. The menu function of the user interface details information that is needed
only for the advanced users. All parts must be read before the device is taken into use.
2 Safety warning
Anyone working with, on or around this equipment should read this manual. Failure
to read, understand and follow the instructions given in this documentation may re-
sult in damage to the unit, injury to the operating personnel and/or poor equipment
performance.
Any internal adjustment, modification or maintenance to this equipment must be un-
dertaken by a qualified service personnel.
If the equipment must be relocated, make sure it is fixed properly on a support stand
or base and move it on a flat surface. When necessary, move the equipment and
the support stand/base separately.
The use of any hazardous materials in this equipment must be monitored by an in-
dustrial hygienist, safety officer or other suitably qualified individual.
Before proceeding, you should thoroughly understand the installation procedures
and take note of the environmental/electrical requirements.
In this manual, important safety related points will be marked with the following
symbols:
NOTE
Used to direct attention to a specific item.
WARNING
Use caution when needed.
If the equipment is used in a manner not specified by this manual, the protection
provided by this equipment may be impaired.
3 About the product
Esco Medical AVT-1®is an anti-vibration table, intended to be used with high-
magnification microscopes. It isolates the microscope from vibrations generated in the
room where the table is placed and from vibration generated above or below any part of
the table top, that is not part of the AVT platform.

AVT-1® User Manual Rev. 1.8
9
The table consists of a sturdy frame and a tabletop in stainless steel and glass. The
AVT platform is located in the middle of the tabletop and it is floating on the AVT mech-
anism.
The anti-vibration mechanism works as a passive damping device and only partially
damps the vibration that is generated on the AVT platform itself.
The microscope must be placed on the anti-vibration float.
In all buildings, floors vibrate at low frequencies that may affect the performance of
high-magnification microscope. The effects of vibration can be greatly reduced by in-
stalling a system that isolates the microscope area. The degree of improvement varies
between instruments and locations, depending on the amplitude and frequency of floor
vibrations.
This product does not carry a CE mark. Only those product categories that are subject
to specific CE marking directives are required (and allowed) to carry CE marking. There
are no such categories or directives that would include a non-electrical anti-vibration
table as AVT-1®. The product is manufactured under ISO 9001 Quality Management
System.
Personal Protective Equipment (89/686/EEC) and Machine Directive (2006/42/EC) is
not applicable for AVT-1®.
4 Accessories supplied
•1 manual
•1 level
•Cable attachments
•Gloves for installation
5 Safety symbols and labels
AVT-1®has a label for type which is placed on the upper left-hand side cross bar. User
labels are shown below.

AVT-1® User Manual Rev. 1.8
10
1.
2.
3.
4.
5.
4.
6.
Table 5.1 Labels
Description
Image
1. Model
2. CE mark
3. Manufacturers address and country
of origin
4. Logo and serial number
5. Observe WEEE
6. Year of manufacture
6 Important safety instructions and warnings
6.1 Before installation
1. Do not use the product if the package is damaged. Contact Esco Medical or the local
representative.
2. Read the User manual completely before use.
3. Always keep these instructions easily accessible near the device.
6.2 During installation
1. Place this unit on a flat, hard and stable surface.
2. Never place the unit on a carpet or similar surfaces.
3. This unit is intended for indoor purpose only.
4. Lift the device carefully. All AVT-1®parts are heavy and may require additional
people to move them around.
5. Float elements have to go into the hole on a tabletop –be careful not to squeeze
fingers or drop the element through the hole.
6.3 Post installation
1. Refer all servicing procedures to a qualified service personnel.
7 Getting started
Follow the guidelines in the safety instructions and warnings section.
If the device is going to be used in a clinical setting, clean and disinfect the
device before use. It is not delivered sterile or in a clinically acceptable clean
state. Consult the cleaning instructions section in this manual for the manufac-
turer’s recommended guidelines!
Assembling Esco Medical AVT-1®.
AVT-1® User Manual Rev. 1.8
11
Do not touch the springs or contact points with bare hands. Skin grease will
compromise the performance of vital friction points.
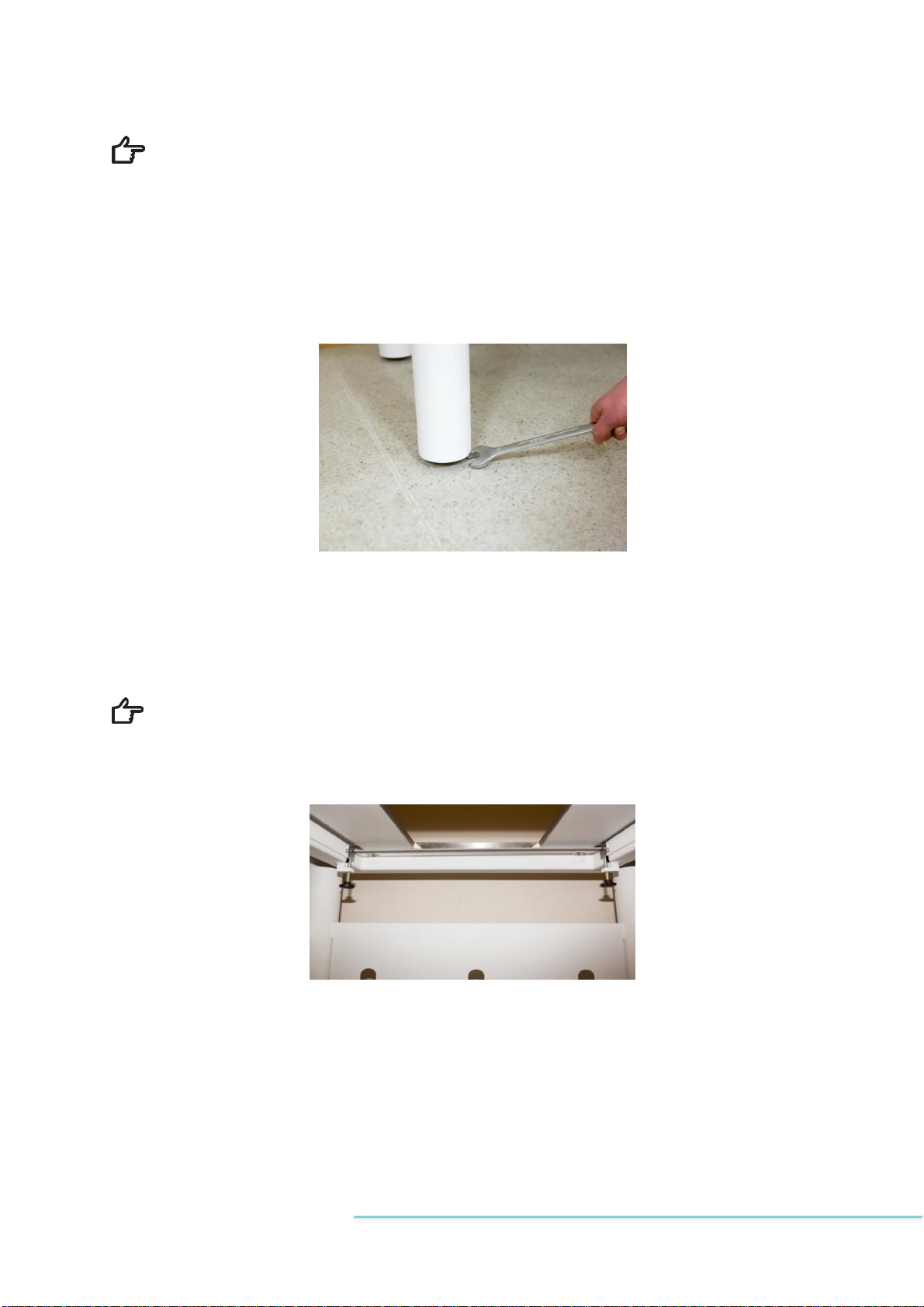
Place the anti-vibration table in an appropriate place on the floor.
Level the table plate horizontally by adjusting table legs with a spanner. In order to en-
sure a good result, place the level on a table plate.
Figure 7.1 Leveling table legs
Put on gloves.
Install the springs into the holes at the front and the back of the table.
A 3rd spring, placed in the centre, may be supplied for the use of a very
heavy microscope. In most cases of normal usage, it is better to mount front and
back springs only.
Figure 7.2 Equal distance from the hole
The float part labeled 1 should be placed on the springs and there should be equal dis-
tance left from the hole on all sides, in order to have space for centering the floating
plate. The holding screws should be facing up.

AVT-1® User Manual Rev. 1.8
12
Figure 7.3 No contact between the floating plate and the table
It is very important that there would be no contact between the floating plate and the
table. Then place the float element 2 on the top of float element 1.
Figure 7.4 Rubber stops facing up
The rubber stops should be facing up. Leave equal distance from the hole on all sides
of the table plate.
Then place the top of the floating element (stainless steel) on the rubber parts again,
leaving equal distance from the hole on all sides of the table plate.
Figure 7.5 Float element is placed too high
The float element should be level with the table top. In the picture above it is too high.
The adjustment can only be done after the microscope has been placed on the float, as
the weight will push it down.

AVT-1® User Manual Rev. 1.8
13
It is the fully laden float that should be level, thus the adjustment is dependent on the
microscope weight.
The float height is adjusted by loosening 4 securing hexagonal head bolts and turning
the handle.
Figure 7.6 Adjusting the float height
After adjustment, tighten the four securing bolts again. If this is done improperly, the
table will go out of adjustment while in use.
Any additional equipment may now be installed.
Make sure that all cables that are connected to the microscope hang freely between the
attachment point on the microscope and the other side. This can be achieved by strip-
ping the cable to the points and making sure that the cable forms an arch between the
points. If the cable is tight or touches area between the anchor points, vibration may be
transferred via the cable.
After successful installation and device testing, complete user training and instructions.
8 Cleaning instructions
Always perform cleaning procedures locally; for more guidance consult ei-
ther your manufacturer or the distributor.
Switch off the devices on the table and remove the mains leads. Clean the surfaces of
AVT-1®with a 70% alcohol solution, using a lint-free cloth.
A list with approved cleaning liquids can be supplied on request.

AVT-1® User Manual Rev. 1.8
14
9 Maintenance
Esco Medical AVT-1®is designed to be easy to use and requires almost no mainte-
nance at all.
Over time, adjustments may become necessary.
The rubber paste should be changed every 2 years, the springs –every 6 years.
Warranty is considered to be void if service and maintenance procedures
are not followed.
Warranty is considered to be void if service and maintenance procedures
are done not by a trained and authorized personnel.
10 User troubleshooting
Table 10.1 Heating system
Vibrations
Cause
Action
The float is touching the table
plate and vibrations are trans-
ferred directly to the microscope.
The floater should be in the
centre position of the hole.
Air should be all around the
floater.
Loose tightening bolts.
Tighten four tightening bolts.
The spring system is not
painted and has to be
completely grease free to
function.
The surface is made to be
rough intentionally.
Grease or oil on the surface of
contact points.
Clean the area with 70% al-
cohol.
Tight cables of micro-
manipulators or any other
equipment that are placed
on the table top will result
in vibrations being trans-
mitted directly to the mi-
croscope.
Cables routed incorrectly.
Tie cables so that they would
not touch the table and would
hang loose between the at-
tachment points.
Microscope is placed unevenly
on the floater.
The weight of the microscope
has to be distributed evenly
on the floater. Since the back
part of a microscope is the
heaviest, it should be moved
more to the front of the table
plate.

AVT-1® User Manual Rev. 1.8
15
11 Specifications
Table 11.1 AVT-1®specifications
Technical specifications
AVT-1®
Overall dimensions (W x D x H)
1200 ×800 ×800 mm
Weight
70 kg
Material
Powder painted mild steel/Stainless steel/Glass
Float
Size 540 ×340
Power consumption
115V 60Hz OR 230V 50Hz
Recommended load weight
26-32 kg
Damping coefficient (6 Hz)
~0.1
Amplitude (6 Hz)
<1 µm
The spectrum of the vertical vibration velocity with the additional mass of 47 kg. Reso-
nance frequency is 5-5.29 Hz.
Figure 11.1 Resonance frequency
Table of contents
Other Esco Medical Medical Equipment manuals

Esco Medical
Esco Medical Mini MIRI Dry User manual

Esco Medical
Esco Medical Multi-zone ART Workstation User manual

Esco Medical
Esco Medical MIRI AVT User manual

Esco Medical
Esco Medical MIRI II-12 User manual

Esco Medical
Esco Medical CE 0123 User manual

Esco Medical
Esco Medical CultureCoin User manual

Esco Medical
Esco Medical MIRI GA User manual