Esco Medical MIRI II-12 User manual

MIRI® II-12 Multiroom
IVF Incubators
For research
use only
MIRI®II-12 Multiroom IVF incubators User Manual Rev. 4.0
2
Es
Tel 215-441-9661 Fax 484-698-7757
Tel +65 6542 0833 Fax +65 6542 6920
The information in this manual and the accompanying product is copyrighted and all
rights are reserved by Esco.
Esco reserves the right to make periodic minor design changes without obligation to
notify any person or entity of such change.
Caution: Federal law restricts this device to sale by or on a licensed healthcare
practitioner's order.
Only to be used by a trained and qualified professional. The device is sold under
exemption 21 CFR 801 Subpart D.
“Material in this manual is provided for informational purposes only. The contents and the
product described in this manual (including any appendix, addendum, attachment or
inclusion) are subject to change without notice. Esco makes no representations or
warranties as to the accuracy of the information contained in this manual. In no event shall
Esco be held liable for any damages, direct or consequential, arising out of or related to the
use of this manual.”

MIRI®II-12 Multiroom IVF incubators User Manual Rev. 4.0
3
Unpacking and Inspection
Claims
Standard Terms and Conditions
Refunds & Credits
Return
Procedure
Return Procedure
prepaid
Restocking Charges
Certification
Warranty and Product Support

MIRI®II-12 Multiroom IVF incubators User Manual Rev. 4.0
4
•
•
•
•
•
When you return an instrument to Esco Medical for service, repair or calibration, we
recommend shipment using the original shipping foam and container. If the original
packing materials are not available, we recommend the following guide for repackaging:
•
•
•

MIRI®II-12 Multiroom IVF incubators User Manual Rev. 4.0
5
Re-calibration of instruments, which have a recommended annual calibration frequency,
is not covered under warranty.
Warranty Disclaimer
In all cases, breaking the tamper-resistant Quality Seal should be avoided at all costs, as
this seal is key to your original instrument warranty. In an event where the seal must be
broken to gain internal access to the instrument, you must first contact Esco Medical Ltd.
You will be required to provide us with the serial number for your instrument, as well as
a valid reason for breaking the Quality Seal. You should break this seal only after you have
received factory authorization. Do not break the Quality Seal before you have contacted
us! Following these steps will help ensure that you will retain the original warranty on
your instrument without interruption.
WARNING

MIRI®II-12 Multiroom IVF incubators User Manual Rev. 4.0
6
Table of contents
..........................................................................................................................
...........................................................................................................................................
......................................................................................................................................
....................................................................................................................................
........................................................................................................
.....................................................................................................
............................................................
............................................................................................................
............................................................................
................................................................................................................................................
...........................................................................................
.....................................................................................................................
.................................................................................
...........................................................................................................................
...........................................................................................................................
................................................................................................................................
...........................................................................................................................................
...................................................................................................................................
......................................................................................................................................
.....................................................................................................................................
..........................................................................................
..........................................................................................................................................
......................................................................................
..................................................................................................................
............................................................................
...............................................................................
...................................................................................................................................
...............................................................................................................
...................................................................................................................
........................................................................................
...........................................................................................................

MIRI®II-12 Multiroom IVF incubators User Manual Rev. 4.0
7
.............................................................................................................
..............................................................................................................
........................................................................................................................................................
.....................................................................................................................
.............................................................................................................................
...............................................................................................................................
..................................................................................................................................
.....................................................................................................................
...............................................................................................................
.................................................................................................................
............................................................................................................................
..............................................................................................................................
.....................................................................................................................
.................................................................
.....................................................................................................................................................
............................................................................................................................
...............................................................................................................................
...................................................................................................................................................
..........................................................................................................................................
.............................................................................................................................
.............................................................................................................................................
...............................................................................................
.................................................................................................................
....................................................................................................................
.............................................................................................................
...........................................................................................................................
......................................................................................
..............................................
.......................................
.....................................................................................................................
........................................................................................................................................
.......................................................................................................................
...............................................................................................................................

MIRI®II-12 Multiroom IVF incubators User Manual Rev. 4.0
8
..............................................................................................
..........................................................................................
............................................................................................................................................
........................................................................................................................
............................................................................................................................
...........................................................................................................................................
.........................................................................................................
.............................................................................................................................
...............................................................................................................
............................................................................................................................
......................................................................................................................
.......................................................................................
................................................................
...................................................................................................................................
...................................................................................................................................
.................................................................................................................
...................................................................................
........................................................................................................................................................
................................................................................................................................
.................................................................................................................................
..................................................................................................................................
....................................................................................................................................
...............................................................................................................
..................................................................................................................
.................................................................................................................................
.....................................................................................................
........................................................................................................
.......................................................................
................................................................................
...................................................................................................................
..........................................................................................................................................
........................................................................................................
.........................................................................................

MIRI®II-12 Multiroom IVF incubators User Manual Rev. 4.0
9
..........................................................................................................................
....................................................................................................
................................................................................................................................................
.......................................................................................................................
.....................................................................................................
........................................................................................................
...............................................................................................................
..................................................................................................................
............................................................................................................................................
.......................................................................................................................
.............................................................................................................
..................................................................................................
.....................................................................................................
...........................................................................................................................................
........................................................................................................................................
..............................................................................................................................................
.......................................................................................................................................
.........................................................................................................................
.......................................................................................................................
..........................................................................................................................................
.................................................................................................................................
....................................................................................................................
...............................................................................................
..................................................................................................
.........................................................................................................................
..........................................................................................................................
..............................................................................................................................
.........................................................................................................................
...........................................................................................................
..........................................................................
............................................................................................
....................................................................................................................................
....................................................................................................................

MIRI®II-12 Multiroom IVF incubators User Manual Rev. 4.0
10
1 How to use this manual
The manual is designed to be read by sections and not ideally from cover to cover. It means
that if the manual is read from start to finish, there will be some repetition and overlap.
: first, familiarize yourself
with the safety instructions; then, proceed to the essential user functions needed for
operating the equipment on a day to day basis; then, review the alarm functions. The menu
function of the user interface details information that is needed . All
parts must be read before the device is taken into use.
2 Safety warning
•Anyone working with, on or around this equipment should read this manual. Failure
to read, understand, and follow the instructions given in this documentation may
damage the unit, injure operating personnel, and/or poor equipment performance.
•Any internal adjustment, modification or maintenance to this equipment must be un-
dertaken by qualified service personnel.
•If the equipment must be relocated, ensure it is appropriately fixed on a support
stand or base and move it on a flat surface. When necessary, move the equipment and
the support stand/base separately.
•The use of any hazardous materials in this equipment must be monitored by an in-
dustrial hygienist, safety officer or other suitably qualified individuals.
•Before you proceed, you should thoroughly understand the installation procedures
and note the environmental/electrical requirements.
•In this manual, important safety-related points will be marked with the following
symbols:
NOTE
It is used to direct attention to a specific item.
WARNING
Use caution.
•If the equipment is used in a manner not specified by this manual, the protection
provided by this equipment may be impaired.
3 Indication for use
The Esco Medical MIRI®family`s multiroom IVF incubators are intended to be used to pro-
vide a stable culture environment at or near body temperature and CO2/N2or premixed

MIRI®II-12 Multiroom IVF incubators User Manual Rev. 4.0
11
gases and humidification for the development of gametes and embryos during in vitro
fertilization (IVF) / assisted reproduction technology (ART) treatments.
4 About the product
Esco Medical MIRI®II-12 multiroom IVF incubator is a CO2/O2incubator.
Direct warming of the dishes in the chambers gives superior temperature conditions in
comparison to conventional multiroom IVF incubators.
The temperature in the compartment will remain stable up to 1 °C (even when a lid is open
for 30s) and will recover within 1 min after the lid is closed.
MIRI®II-12 multiroom IVF incubator has 12 completely separate culture heat chambers.
Each chamber has its own heated lid and warming plate for Petri dish. The MIRI® II-12
maximum capacity is 48 pcs 35 mm a 60 mm Petri dishes and 12 pcs 4-well Petri dishes
To ensure maximum performance, the system of the MIRI® II-12 multiroom IVF incubator
have 24 completely separate PID temperature controllers. They control and regulate
temperature in culture chambers and lids. Compartments do not affect each other`s
temperatures in any way. The top and the bottom of each compartment is separated with a
PET layer so that the lid temperature would not affect the bottom. For validation purposes,
each compartment has a PT-1000 sensor built in. The circuitry is separated from the unit`s
electronics so it remains a truly separate validation system.
The multiroom IVF incubator has to be supplied with 100% CO2and 100% N2in order to be
able to control the CO2and O2concentrations in the culture chambers.
A dual beam infrared CO2sensor with extremely low drift rates controls the CO2level. A
chemical medical grade oxygen sensor controls the level of O2.
Gas recovery time is less than 3 min. after opening the lid. To validate gas concentration, the
MIRI® II-12 multiroom IVF incubator is fitted with 12 gas sample ports that allow the user
to sample gas from the individual compartment.
The multiroom IVF incubator features a recirculated gas system where gas is continuously
put into the compartment and taken out at the same rate. Gas is cleaned via 254 nm UVC
light with direct gas contact between the bulb and gas, then through a VOC filter and through
a HEPA filter. The UVC light has filters that inhibit any 185 nm radiation that would produce
dangerous ozone. The VOC filter is located under the UVC light.
Complete gas repletion in the system takes less than 5 min.

MIRI®II-12 Multiroom IVF incubators User Manual Rev. 4.0
12
The total gas consumption is very low. Less than 2 l/h CO2and 5 l/h N2in use.
For safety reasons the multiroom IVF incubator has a very complete gas control system that
consists of: pressure regulator (preventing dangerous gas pressure problems), gas flow
sensors (actual consumption can be accumulated), gas pressure sensors (then user knows
that the pressure and variation can be logged to avoid dangerous conditions), gas filters (to
avoid valve problems).
Petri dish location in a compartment is easy to reach and safe because of the compartment
numbering and the ability to write on the white lid with a pen.
The multiroom IVF incubator has been primarily developed and designed for incubation of
gametes and embryos with an overlay of either Paraffin or mineral oil.
The multiroom IVF incubator has a built-in PC which operates the Esco Medical Data logger
software for long-term data logging and data storage.
USB module enables the QC data to be transferred for off-site evaluation by performing
this, the manufacturer can provide a valuable service to the customers (MIRI® II-12).
A pH sensor port is part of the DAQ package. The user can plug any standard BNC pH probe
to the unit and measure the pH in the samples at will.
The device is manufactured under a full EU certified 13485 ISO quality management
system.
This product fulfils the requirements of EN60601-1 3rd edition standards as a Class I type
B equivalent device suited for continuous operation. It also conforms to the requirements of
the Regulation (EU) 2017/745 concerning medical devices and is classified as a Class IIa
device under rule II.
Personal Protective Equipment (89/686/EEC) and Machine Directive (2006/42/EC) is not
applicable for the MIRI®II-12 multiroom IVF incubators. Also, the MIRI®II-12 multiroom
IVF incubators does not contains or incorporates: a medical substance, including a human
blood or plasma derivate; tissues or cells, or their derivates, of human origin; or tissues or
cells of animal origin, or their derivatives, as referred to in Regulation (EU) No. 722/2012.
5 Transport, Storage and Disposal
5.1 Transportation requirements
The device is packed in a carton box, and it is wrapped in polyethylene. The box is affixed to
a pallet with special straps.

MIRI®II-12 Multiroom IVF incubators User Manual Rev. 4.0
13
A visual inspection should be done if there is any damage. If no damage is found, the MIRI®
II-12 multiroom IVF incubator can be prepared for transport.
•Label with the marked packing date
•Label with the product name and serial number
5.2 Storage and operation environment requirements
5.2.1 Storage requirements
•The unit can be in storage for one year. If stored longer than one year, the unit must
be returned to the manufacturer for a new release test
•The unit can be stored at temperatures between -20 °C and + 50 °C
•Keep away from direct sunlight
•Caution: consult the accompanying documents for important safety-related
information such as warnings and precautions that cannot be presented on the
device itself for various reasons
•Do not use if the packing material is damaged
•Keep dry
5.2.2 Operation environment requirements
•Environmental temperatures below 30 °C
•Away from direct sunlight
•Kept dry
•For indoor use only
5.3 Disposal
Information on handling of the unit as per the WEEE Directive (Waste Electrical and Elec-
tronic Equipment).
The device may have been used for treating and processing infectious substances.
Therefore, the device and device components may be contaminated. Prior to disposal,
the whole device must be disinfected or decontaminated.
The unit contains reusable materials. All components (except for the VOC/HEPA and HEPA
filters) can be discarded as electrical waste after cleaning and disinfection.

MIRI®II-12 Multiroom IVF incubators User Manual Rev. 4.0
14
Please note that the VOC/HEPA and HEPA filters must be discarded following the applicable
national regulations for special solid waste.
6 Supplied service parts and accessories
Service parts:
•1 VOC/HEPA filter capsule
•2 HEPA filters for input gas supply
•12 warming blocks
•4 warranty labels
•1 USB stick containing a PDF version of the user manual
•1 medical grade power cord
•1 3.5mm external alarm jack connector
•1 set of fast male connectors with 15 silicone pipes
Accessories do not apply with the MIRI®II-12 multiroom IVF incubator.
7 Safety symbols and labels
There are several user labels on the surface of MIRI®II-12 multiroom IVF incubator to guide
the user. User labels are shown below.
Table 7.1 Packing box and electrical safety labels
Description
Image
Packing box label:
1. If stored longer than the shelf life, the unit must be
returned to the manufacturer for a new release test.
2. Shipping temperature between -20 °C and +50 °C.
3. Keep away from direct sunlight.
4. Caution: consult the accompanying documents for
important safety-related information such as warn-
ings and precautions that cannot, for a variety of rea-
sons, be presented on the device itself.
5. Consult instructions for proper use of the device.
6. Do not use if the packing material is damaged.
7. Rx Only.
8. Keep dry.
1. Consult instruction for use.
2. Warning on the back of the device indicates that an
earth connection is needed and the mains information
electrical shock (never remove any cover).
MIRI®II-12 Multiroom IVF incubators User Manual Rev. 4.0
15
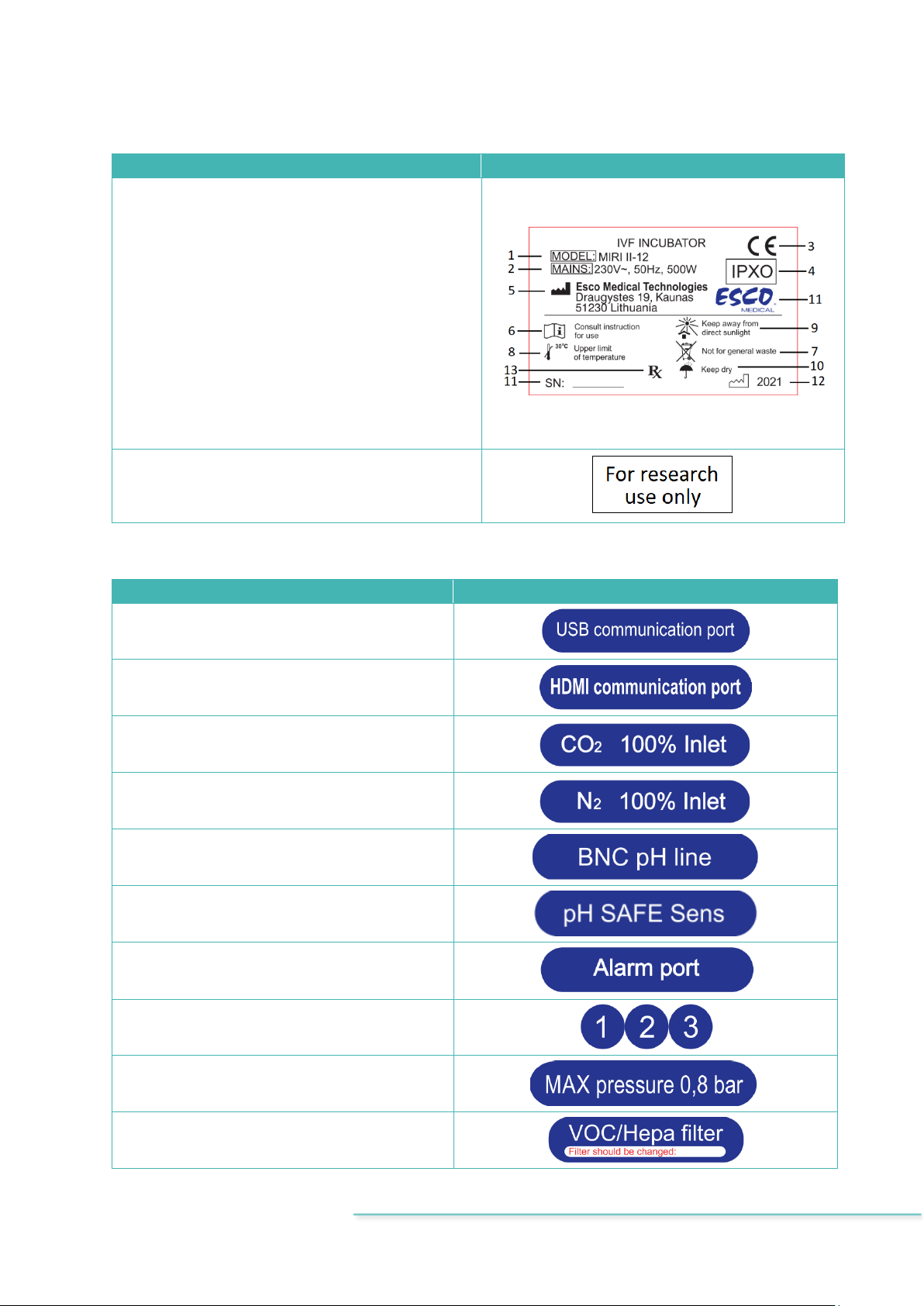
Table 7.2 Device labels
Description
Image
1. Model.
2. Mains power rating.
3. CE mark.
4. Not protected against the ingress of water.
6. View instruction for use.
7. Observe WEEE.
8. Upper limit of temperature.
9. Keep away from direct sunlight.
10. Keep dry.
11. Logo and serial number.
12. Year of manufacture.
13. Rx only.
1. For research use only.
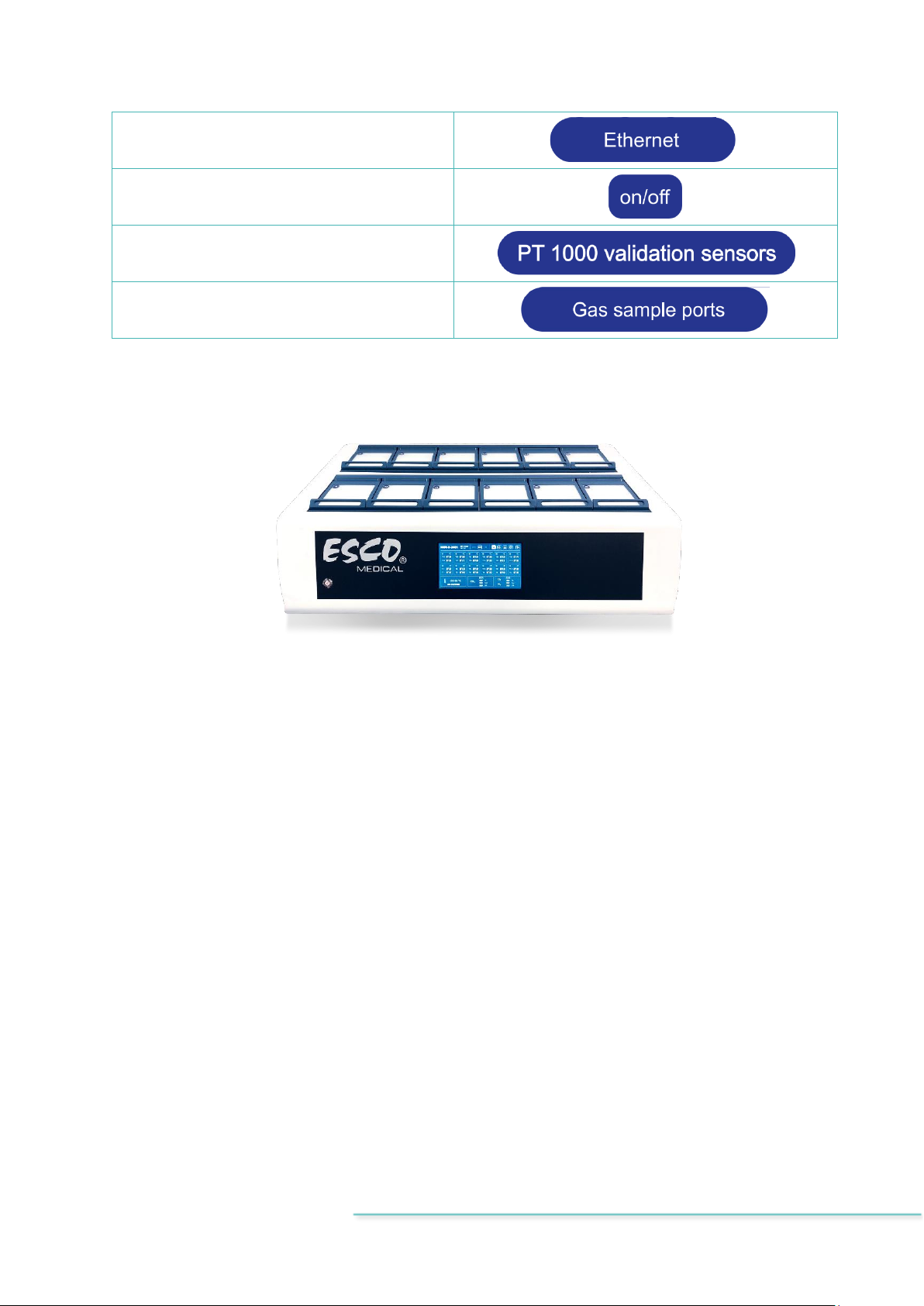
Table 7.3 Labels on the MIRI®II-12 multiroom IVF incubator
Description
Image
USB communication port
HDMI communication port
CO2inlet
N2inlet
BNC pH line
pH SAFE Sense
Alarm port
Compartment numbers are indicated on the
top corner of the lid with a label
Maximum pressure 0.8 bar
VOC/HEPA filter
MIRI®II-12 Multiroom IVF incubators User Manual Rev. 4.0
16
Ethernet
PC on/off
PT 1000 validation sensors
Gas sample ports
Compartment numbers are shown in the picture below and also indicated on the top of the
lids with labels:
Figure 7.1 Compartment numbers on MIRI®II-12 multiroom IVF incubator
8 Important safety instructions and warnings
8.1 Before installation
1. Do not use the product if the package is damaged. Contact Esco Medical or the local
representative.
2. Read the user manual thoroughly before use.
3. Always keep these instructions easily accessible near the device.
8.2 During installation
1. Never place this unit on top of other equipment that gives off heat.
2. Place this unit on a flat, hard and stable surface.
3. Do not place the unit on a carpet or similar surfaces.
4. Do not defeat the safety purpose of the grounding-type (earthing) plug.
5. A grounding-type (earthing) plug with two blades and a third prong are provided for
your safety. If the provided plug does not fit into your outlet, consult an electrician to
replace the outlet.
6. Always connect the power cord to a properly grounded outlet and only use the cord
that came with the device.

MIRI®II-12 Multiroom IVF incubators User Manual Rev. 4.0
17
7. Do not install near any heat sources such as radiators, heat registers, stoves or other
apparatus that produce heat.
8. Do not use this device near water sources.
9. Use only 100% concentration CO2and 100% concentration N2gases.
10. Always use an external HEPA filter for input CO2and N2gases.
11. Do not use this product if the room temperature exceeds 30 °C.
12. Place this unit in a location with adequate ventilation to prevent internal heat build-
up. Leave at least 10 cm clearance from the rear, 30 cm from the top and 20 cm from
left and right to prevent overheating and allow access to the ON/OFF switch in the
back.
13. This unit is intended for indoor purposes only.
14. The unit must be connected to a suitable uninterrupted power supply (UPS) source.
8.3 Post installation
1. Refer all servicing procedures to qualified service personnel.
2. Servicing is required according to the service manual as well as cases when the device
has been damaged in any way, e. g. suppose the apparatus has been dropped, exposed
to rain or moisture or does not operate normally. The MIRI®II-12 multiroom IVF in-
cubators contain high voltage components that may be hazardous.
3. Unplug this device during lightning storms or when unused for an extended period
of time.
4. Protect the power cord from being walked on or pinched, particularly at the plug,
convenience receptacles and the point where it exits from the apparatus.
5. Perform temperature and gas calibration at the intervals described in the manuals.
6. Never leave the lids open for more than 10 sec while in use.
7. VOC/HEPA filters must be changed every 3 months.
8. A maintenance plan must be fulfilled to keep the device safe.
9. NEVER block gas supply holes in the compartment.
10. Ensure that CO2and N2gas supply pressures are kept stable at 0.4 0.6 bar (5.80
8.70 PSI).
11. Never use any other except Esco Medical filter. Otherwise, the warranty will be void.
12. Do not use the device without a proper Esco Medical VOC/HEPA filter attached.
9 Getting started
The MIRI®II-12 multiroom IVF incubators must be installed by authorized and
trained personnel only!
1. Follow the guidelines in the safety instructions and warnings section.
2. Connect the mains cable to the UPS.
3. Connect the mains cable to the MIRI®II-12 multiroom IVF incubator.
4. Connect gas lines.
MIRI®II-12 Multiroom IVF incubators User Manual Rev. 4.0
18
5. Set the gas pressure on the external gas regulator at 0.4 0.6 bar (5.80 8.70 PSI).
6. Switch on the MIRI®II-12 multiroom IVF incubator in the back.
7. Observe for standard functionality.
8. Let the unit warm up and stabilize for 20 min.
9. Follow the guidelines in the Validation guide .
10. Complete user training and finish reading instructions.
11. After a burn-in phase of 24 hours, the unit is ready for use IF the testing is successful.
Clean and disinfect the device before use. It is not delivered sterile or in a clini-
cally acceptable cleanliness state. Consult the cleaning instructions section in this
manual for the manufacturer's recommended guidelines!
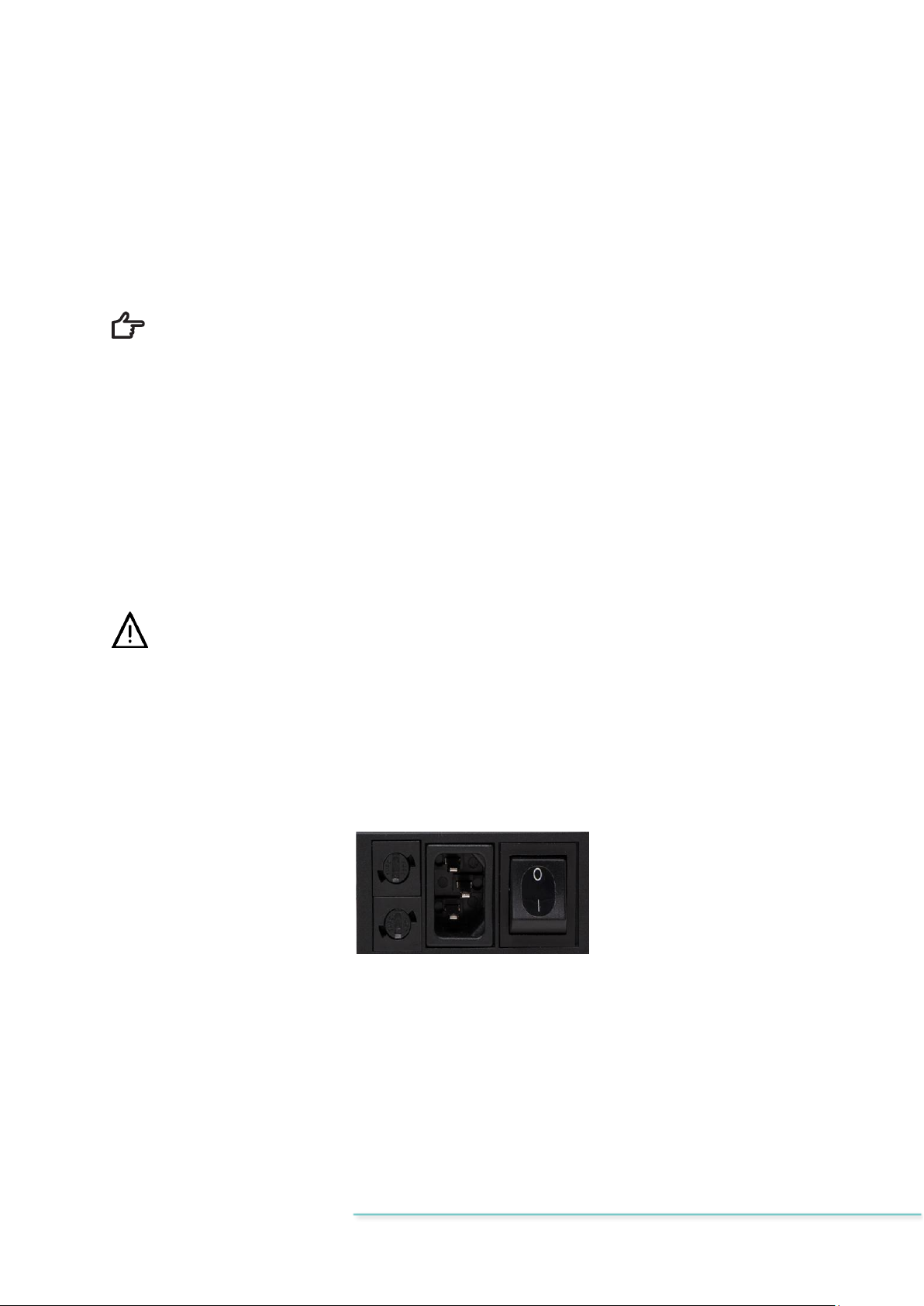
10 Mains connection
The MIRI®II-12 multiroom IVF incubators come with a detachable mains power cord. The
power cord is prepared for the country in which the unit is intended to be used.
The ON/OFF switch provides the user with a means to isolate the MIRI®II-12 multiroom IVF
incubator from the mains.
Do not defeat the safety purpose of the grounding-type plug! A grounding-type
plug has two blades and a prong, which is provided for your safety. If the provided
plug does not fit into your outlet, consult an electrician to replace the outlet.
The power requirement is 230V 50 Hz OR 115V 60Hz. The built-in power supply has a
switch mode that automatically adjusts to the correct mains power between 100V-240V AC
50-60 Hz.
Figure 10.1
11 Gas connections
There are two gas inlets on the back of the unit. These ports are marked "CO2100% Inlet"
and "N2 100% Inlet".

MIRI®II-12 Multiroom IVF incubators User Manual Rev. 4.0
19
Figure 11.1 Gas inlets on the back of the MIRI®II-12
multiroom IVF incubator
CO2inlet should be connected to a 100% concentration of CO2. CO2control in the
compartment is available in the range from 3.0% to 10.0%.
N2inlet should be connected to a 100% concentration N2if low oxygen conditions are
required. O2 control in the compartments is available in the range from 5.0% to 10.0% by
infusing N2gas.
Gas pressure for both inlets should be between 0.4-0.6 bar (5.80 –8.70 PSI), and
it must be kept stable!
.
Figure 11.2 Pressure regulator
Connect CO2gas to the CO2inlet with a suitable silicone tube. Ensure that the tube is fastened
with a clip so that it does not accidentally loosen itself during a sudden pressure fluctuation.
Use the supplied 0.2µ HEPA filter on the gas line just before the inlet on the incubator. Notice
the flow direction.
Connect the N2inlet to the Nitrogen Bottle.
Figure 11.3 Gas filter

MIRI®II-12 Multiroom IVF incubators User Manual Rev. 4.0
20
12 HEPA/VOC filter
VOCs are hydrocarbon-based compounds that are found in fuel, solvents, adhesives and
other compounds. Examples of VOCs include isopropanol, benzene, hexane, formaldehyde,
vinyl chloride.
VOCs can also occur in medical gases, such as CO2and N2. It is essential to use in-line VOC
filters to prevent these fumes from entering your multiroom IVF incubators for your medical
gasses.
Unexpected sources of VOCs are commonly found in IVF labs. These can include cleaning
agents, perfumes, cabinetry, grease on the wheels of equipment and sources in HVAC equip-
ment.
VOCs are typically measured in parts per million (ppm.) They can also be reported in parts
per billion (ppb.) For IVF, the recommended count below 0.5 ppm; the total quantity of VOCs
should be below <0.2 ppm or preferably zero.
High levels of VOCs (over 1 ppm) are toxic to embryos, resulting in poor embryo develop-
ment and even probable failure to reach the blastocyst stage.
VOC levels in the 0.5 ppm range will typically allow an acceptable blastocyst development
and reasonable pregnancy rates but will likely result in a high percentage of miscarriages.
A combined HEPA and VOC filter (carbon filter) are integrated into the construction of the
MIRI®II-12 multiroom IVF incubator. Before entering the multiroom IVF incubator, the gas
is sent through the filter in a single pass. Then, upon return from the compartment, the gas
is filtered again. The recirculation system constantly filters gas in the multiroom IVF incu-
bator.
The combined HEPA and VOC filter are mounted on the device's back to ease access and
replacement.
12.1 Installation of a new filter capsule
Two blue caps that are installed on the filter can be discarded during unwrapping. Correct
Filter element must be changed every 3 months. Mark the date when it is put on
and make sure to keep this interval!
Start by putting the blue fittings on the filter into the filter holder sockets. The flow arrow
on the MIRI®II-12 multiroom IVF incubator and the filter should point in the same direction.
Table of contents
Other Esco Medical Medical Equipment manuals

Esco Medical
Esco Medical CultureCoin User manual

Esco Medical
Esco Medical Mini MIRI Dry User manual

Esco Medical
Esco Medical CE 0123 User manual

Esco Medical
Esco Medical Multi-zone ART Workstation User manual

Esco Medical
Esco Medical MIRI AVT User manual

Esco Medical
Esco Medical MIRI GA User manual

Esco Medical
Esco Medical AVT-1 User manual
Popular Medical Equipment manuals by other brands

LABORIE
LABORIE NXT Go owner's manual

TZ Medical
TZ Medical ARC Instructions for use

Otto Bock
Otto Bock Dyneva 50R300N Instructions for use

OmniPod
OmniPod DASH SYSTEM PODDER Resource Guide

Special Tomato
Special Tomato Out & About Seat user manual
bort medical
bort medical select GenuZip Instructions for use