EN-2
Dear System Owner:
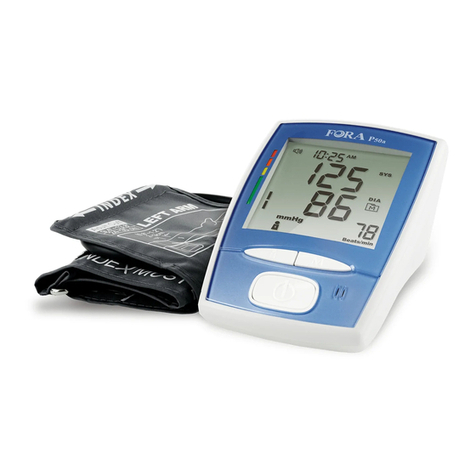
Thank you for purchasing the FORA Vital Blood Pressure
Monitoring System. This manual provides important information
to help you operate this system properly. Before using this
product, please read the following contents thoroughly and
carefully.
With the compact size and easy operation of this FORA Vital
Blood Pressure Monitoring System, you can easily monitor your
blood pressure by yourself at any time or place. In addition,
this system can help you and your healthcare professionals to
monitor and adjust your treatment plans, and keep your blood
pressure under control.
If you have other questions regarding this product, please
contact the place of purchase.
IMPORTANT SAFETY PRECAUTIONS
READ BEFORE USE
1.Use this device ONLY for the intended use described in this
manual.
2.Do NOT use accessories which are not specied by the
manufacturer.
3.Do NOT use the device if it is not working properly or
damaged.
4.Do NOT use under any circumstances on newborns or
infants.
5.This device does NOT serve as a cure for any symptoms or
diseases. The data measured are for reference only. Always
consult your doctor to have the results interpreted.
6.Keep the equipment and its exible cord away from hot
surfaces.
7.Do NOT apply the cu to areas other than the place
directed.
8.Use of this instrument in a dry environment, especially if
synthetic materials are present (synthetic clothing, carpets
etc.) may cause damaging static discharges that may cause
erroneous results.
9.Do not use this instrument in close proximity to sources of
strong electromagnetic radiation, as these may interfere
with the accurate operation.
10.If you experience any serious incident that occurred in
relation to the use of this product, please report it to the
manufacturer and the competent authority of medical
devices in your country.
A serious incident means any incident that directly or
indirectly led, might have led, or might lead to any of the
following:
(a) the death of a patient, user, or other people,
(b) the temporary or permanent serious deterioration of a
patient’s, user’s or other person’s state of health,
(c) a serious public health threat.
KEEP THESE INSTRUCTIONS IN A SAFE PLACE