WARNING
Long-term hyperthermia may blister skin. Also, when producing local
hyperaemia by means of hyperthermia, a certain risk of applying a heat exposure
that is harmful to the skin is always present. For special patients, e.g. patients in
shock, patients with low blood pressure, and patients with vascular constrictions,
particular care should be taken whenever hyperthermia is produced.
l During monitoring, electrodes need to be replaced at least every 4 hours to
avoid burn on patient’s skin.
l
CAUTION
· This device is not intended for use with the IB-7006 of the polygraph system
(MCS-7000 System). Do not use polygraph system modules with the DS-5000
System. The device can be damaged and/or safety cannot be ensured.
· This device is not intended for use outside the hospital (including in ambulance),
under MRI environment, inside a hyperbaric chamber or in the presence of
flammable anesthetic gas.
· For quality improvement, specifications are subject to change without prior notice.
· Upgrading of the program, maintenance test, module install (upgrade), and
demonstration functions should be performed only by Fukuda Denshi authorized
personnel. Users should not attempt to perform any of these operations.
Malfunction may occur to the device.
· Do not open the external case of this device.
· Lead cables and amplifiers are galvanically isolated from other parts of the
equipment, but if the electrodes are touching other metal parts or patient’s limbs, the
galvanic isolation will not function. Electric shock may occur even if the equipment
is grounded.
· If the power cables for several equipments are connected to the same outlet, the
leakage current will increase and may cause hazardous condition to the patient.
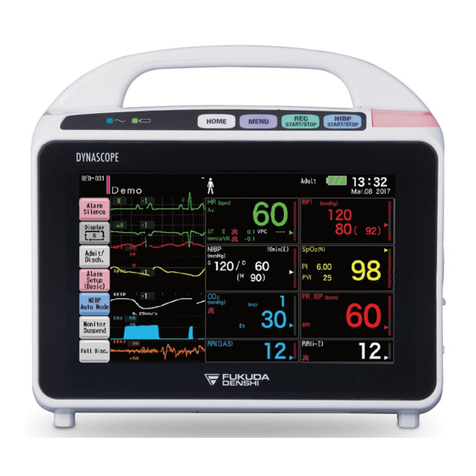
12ECG/RESP Monitoring
· When using the HJ-500 and the HS-500 simultaneously, the measurements by the
HJ-500 will be given priority. Waveforms and measurements measured by the
HS-500 will not be displayed.
· The HJ-500 is a CF type equipment, but is not intended for use directly to the heart.
· Be especially cautious when monitoring ECG of a patient with pacemaker or other
electrical equipment connected.
· For stable monitoring, use the specified electrodes and leads.
· When applying ECG electrodes to the patient, follow the instruction of the physician.
· If ECG electrodes are located at the same place for a long time period, some
patients may experience a rash. Check the electrodes periodically and change
the placement if necessary.
· When using a defibrillator during ECG monitoring, the high-voltage discharge will
generate a polarization potential in the electrode, and may interrupt monitoring for a
few seconds.
· A pacemaker pulse is detected by hardware. Stable detection may not be acquired
if the pacemaker pulse signal gets small depending on the pacemaker type, output
pulse voltage, pulse width, electrode lead type (unipolar, bipolar), and lead position.
· If there is a device generating noise near the patient, this noise can be erroneously
detected and displayed as a pacemaker pulse.
· When a pacemaker pulse wave resembles the QRS wave, QRS detection (heart rate
counting) may not be performed.
v