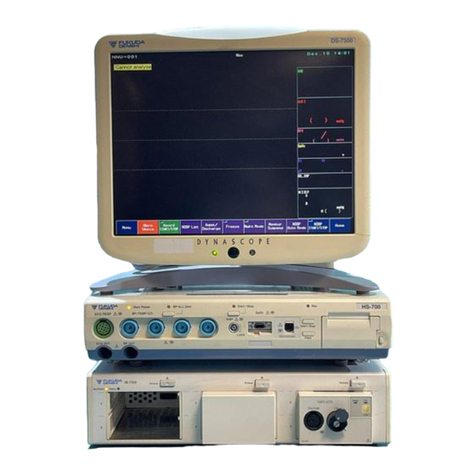
Preface
Thank you for purchasing our product.
Before using this product, read the following precautions to make sure the
product is used correctly and safely.
Safety ··············································································· ii
About the Safety Precautions ·······································ii
The Meaning of Each Safety Precaution················· ii
Warning Labels Attached to the Unit······················· ii
Measurement Unit for Each Parameter ······················· iii
Graphic Symbols ··················································· vi
Precautions for Safe Operation of Medical Electrical
Equipment ································································· viii
Precautions about the Maintenance ···························· ix
Precautions about the Network System························x
Medical Telemetry···················································x
Bidirectional Wireless Communications Module
(TCON) (Only for USA)······································· xi
Precautions when Using with Other Equipment ········ xiii
Pacemaker··························································· xiii
Non-Explosion Proof ············································ xiii
Defibrillator··························································· xiii
Electrosurgical Instrument ··································· xiv
MRI (Magnetic Resonance Imaging)···················· xiv
Precautions about Connections to Peripheral
Devices······························································ xv
Precautions for Using the Equipment ························ xvi
This System ························································· xvi
Wired Network (DS-LANII/ DS-LANIII)··············· xxiv
Wireless Network System ··································· xxv
RTC and Data Backup ········································ xxv
Precautions about the Ventilator Monitoring············ xxvi
Precautions about the SpO2Sensor························ xxvi
Precautions about the NIBP Cuff····························· xxvi
Precautions about Disposing of the Equipment,
Accessories, or Components··································· xxvi
Precautions about Transportation····························xxvii
Monitoring after Power Failure ································xxvii
To Prepare for Emergency Use ·······························xxvii
Electromagnetic Compatibility ································xxviii
Precautions for Safe Operation under
Electromagnetic Influence ·····························xxviii
EMC Guidance ·················································xxviii
i