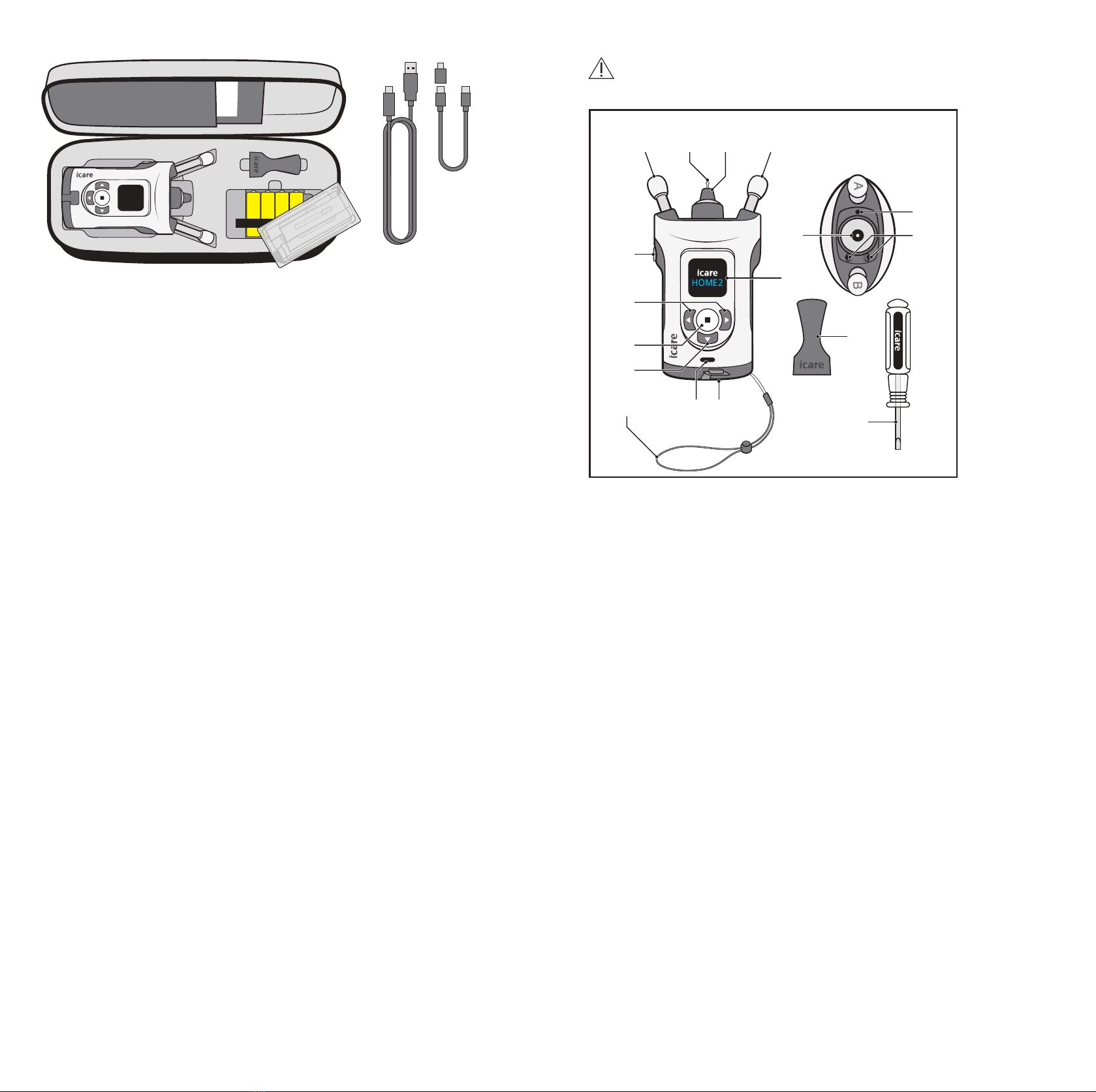
6 7
WARNING! Do not connect anything to the tonometer’s USB port but the USB cable
supplied with the tonometer.
WARNING! Keep the USB cable out of the reach of children and pets due to the risk of
strangulation.
WARNING! The tonometer’s batteries are not rechargeable. Do not try to charge the
tonometer with USB chargers connected to a mains voltage.
WARNING! Do not connect the USB cable to the tonometer’s USB port except when
uploading patient measurement data. Do not take any measurements when the USB
cable is connected.
WARNING! The tonometer should only be opened by qualified iCare service personnel.
The tonometer does not contain any user-serviceable parts, apart from the batteries
other than changing the batteries at least annually and the probe base every six months.
If there is a reason to believe that the servicing of the tonometer is necessary, contact
the manufacturer or local distributor.
WARNING! The tonometer must not be repaired or re-assembled by any other than the
manufacturer or its authorized service center. If the tonometer is broken, do not use it.
Take it to an authorized iCare service center for repair.
WARNING! To avoid possible damage, keep the tonometer out of the reach of children
and pets. The probe base, battery cover, screws, collar, and probes are small objects and
may be accidentally swallowed.
WARNING! Do not change the batteries or the probe base when the USB cable is
connected.
WARNING! Servicing or maintenance actions must not be performed while the
tonometer is in use.
WARNING! The tonometer must be switched off when the probe base is changed.
WARNING! The probe base must be changed, not cleaned.
WARNING! Never immerse the tonometer in liquid. Do not spray, pour, or spill liquid
Immediately remove any liquid from the surface of the tonometer.
WARNING! Do not modify the tonometer in any way. Changes or modifications not
expressly approved by the manufacturer could void the user’s authority to operate the
tonometer.
WARNING! Use of this equipment adjacent to or stacked with other equipment should
be avoided because it could result in improper operation. If such use is necessary, this
equipment and the other equipment should be observed to verify that they are
operating normally.
WARNING! Use of accessories, transducers, and cables other than those specified or
provided by the manufacturer of this equipment could result in increased
electromagnetic emissions or decreased electromagnetic immunity of this equipment
and result in improper operation.
WARNING! Interference may occur in the vicinity of equipment marked with the
non-ionizing radiation symbol.
WARNING! Sources of power frequency magnetic field should be used no closer than
manufacturer, to avoid the degradation of performance.
WARNING! Portable RF communications equipment (including peripherals such as
antenna cables and external antennas) should be used no closer than 30 cm (12 inches)
to any part of the tonometer, including the cables specified by the manufacturer, to
avoid the degradation of performance.
PRECAUTION! Read this manual carefully, as it contains important information about
using and servicing the tonometer.
PRECAUTION! Use the tonometer only for measuring intraocular pressure. Any other use
is improper. The manufacturer is not liable for any damage arising from improper use,
PRECAUTION! Do not use the tonometer near inflammable substances, including
inflammable anesthetic agents.
PRECAUTION! Report any serious incidents related to the tonometer to your competent
health authority and the manufacturer or the manufacturer’s representative.
PRECAUTION! When removing the tonometer from its packaging, and each time before
use, visually inspect the tonometer for any external damage, particularly for possible
damage to the tonometer casing. If you suspect damage to the tonometer, contact the
manufacturer or the distributor of the tonometer.
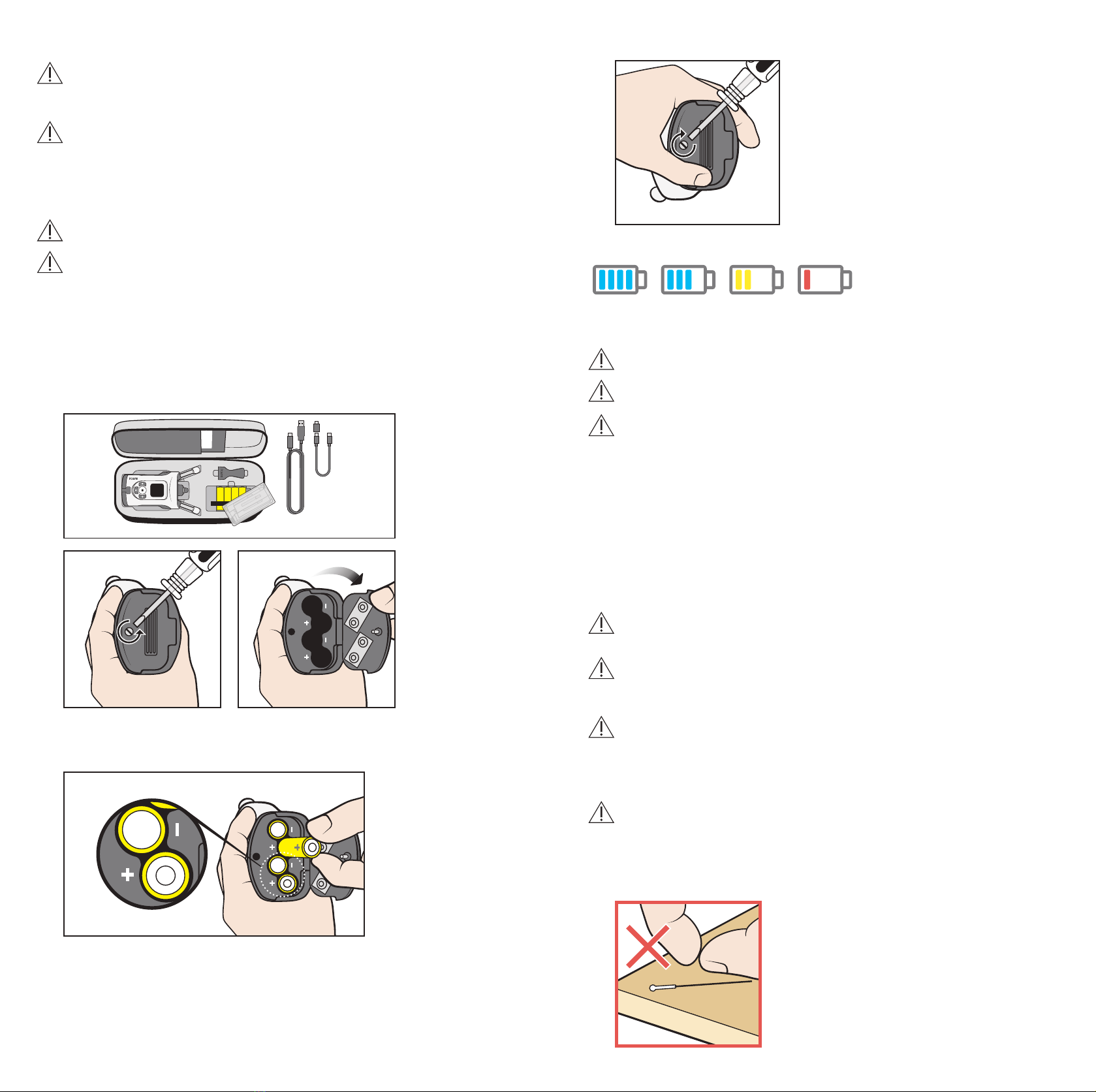
PRECAUTION! Use only the battery type specified in the technical specification section
of this manual. Do not use rechargeable batteries, as they do not have sufficient voltage.
PRECAUTION! The tonometer switches off the display when it has not detected any
movement for 15 seconds. The tonometer switches off automatically if it has not been
used for 3 minutes.
PRECAUTION! Before taking measurements, update the tonometer’s time to your local
time manually from the tonometer’s settings or automatically by connecting the
tonometer to the iCare PATIENT2 application or to the iCare EXPORT software.
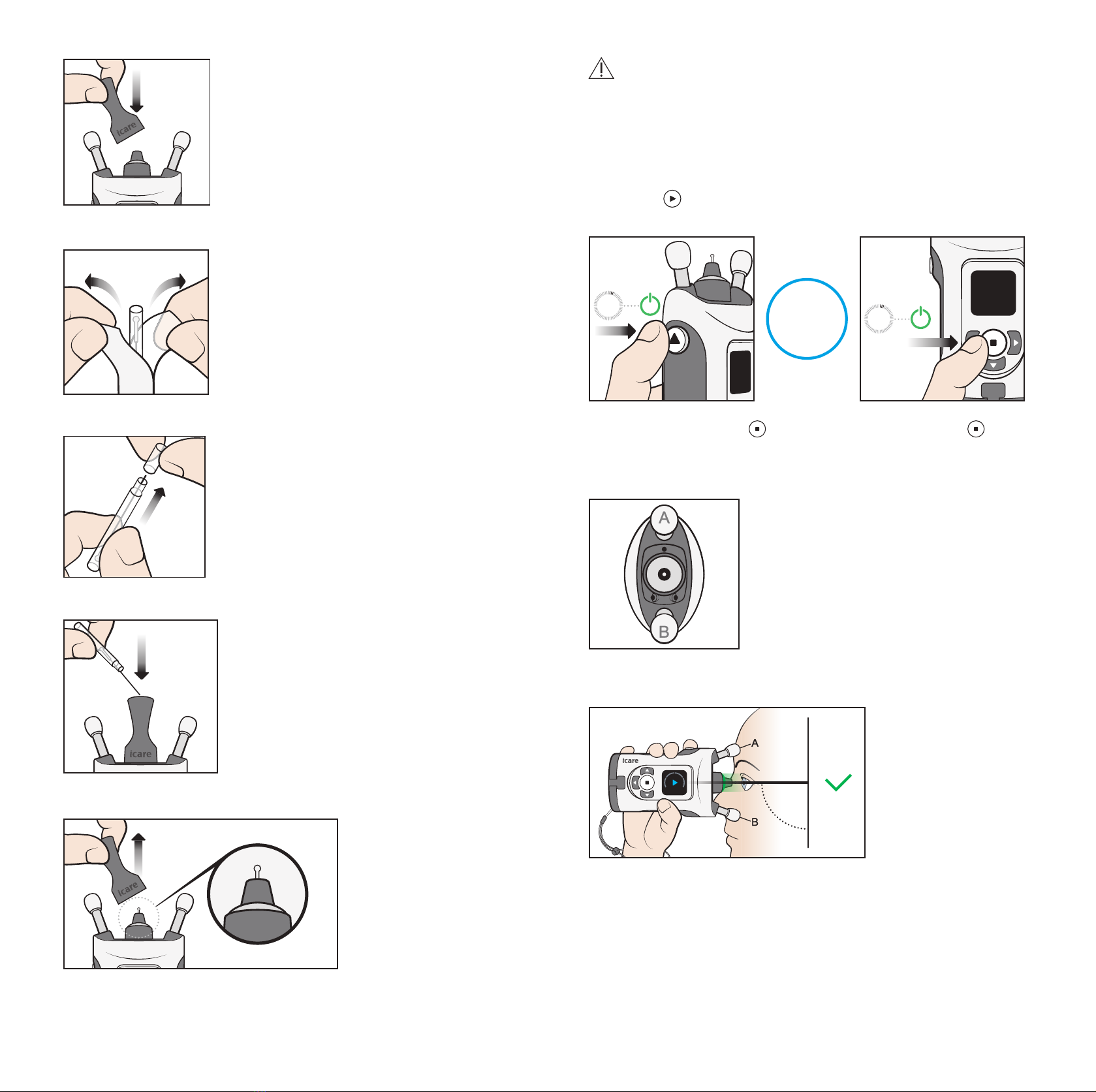
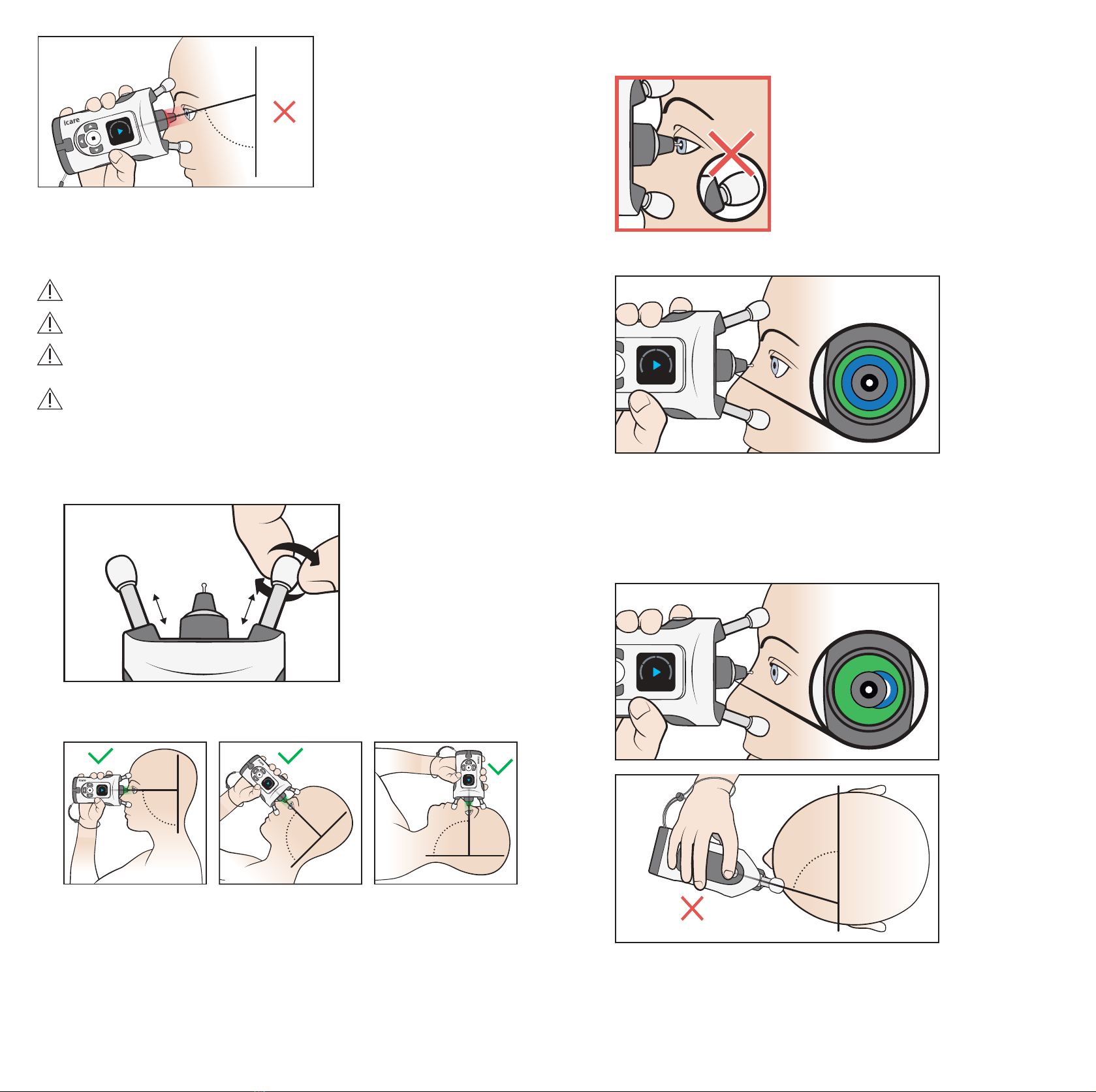
PRECAUTION! Do not cover the eye recognition transmitters or sensor during the
measurement, for example with your fingers. Keep your hand, hair, and objects such
that causes an error.
PRECAUTION! Eye detection is based on the difference of infrared reflections received
from the transmitters: the nose side reflects more than the temple side. If the
transmitters become dirty, the recognition may be interfered.
PRECAUTION! To ensure the correct functioning of the tonometer, change the probe
base every six months.
PRECAUTION! Non-ME equipment (computer or mobile device) used in the system
requirements for multimedia equipment: CISPR 32 and CISPR 35.
PRECAUTION! The measurement method of the tonometer is based on a magnetically
induced motion of a probe and therefore an external magnetic or radiated RF
electromagnetic field disturbing the probe may prevent the measurement. In such a
case the tonometer continuously displays error messages during measurement and asks
you to repeat the measurement. Situation can be solved either by removing the source
of interference from the vicinity of the tonometer or by performing the measurement in
different location with no such interference.
PRECAUTION! The measurement data transfer may be interrupted during electromagnetic
data is transferred successfully.
PRECAUTION! Portable and mobile RF communications equipment can affect the
tonometer.
PRECAUTION! Although the tonometer’s own electromagnetic emissions are well below
the levels permitted by the relevant standards, they may cause interference in other
nearby devices, for example sensitive sensors.