1
AMENDMENTS TO INSTRUCTION MANUAL
JEM-1200EX
No. CI1EM1200EX-2
(EM157012)
The instrument has undergone certain modifications in order to improve performance and facilitate
operation. Please, therefore, note the following changes and correct your manual accordingly. We regret any
inconvenience caused.
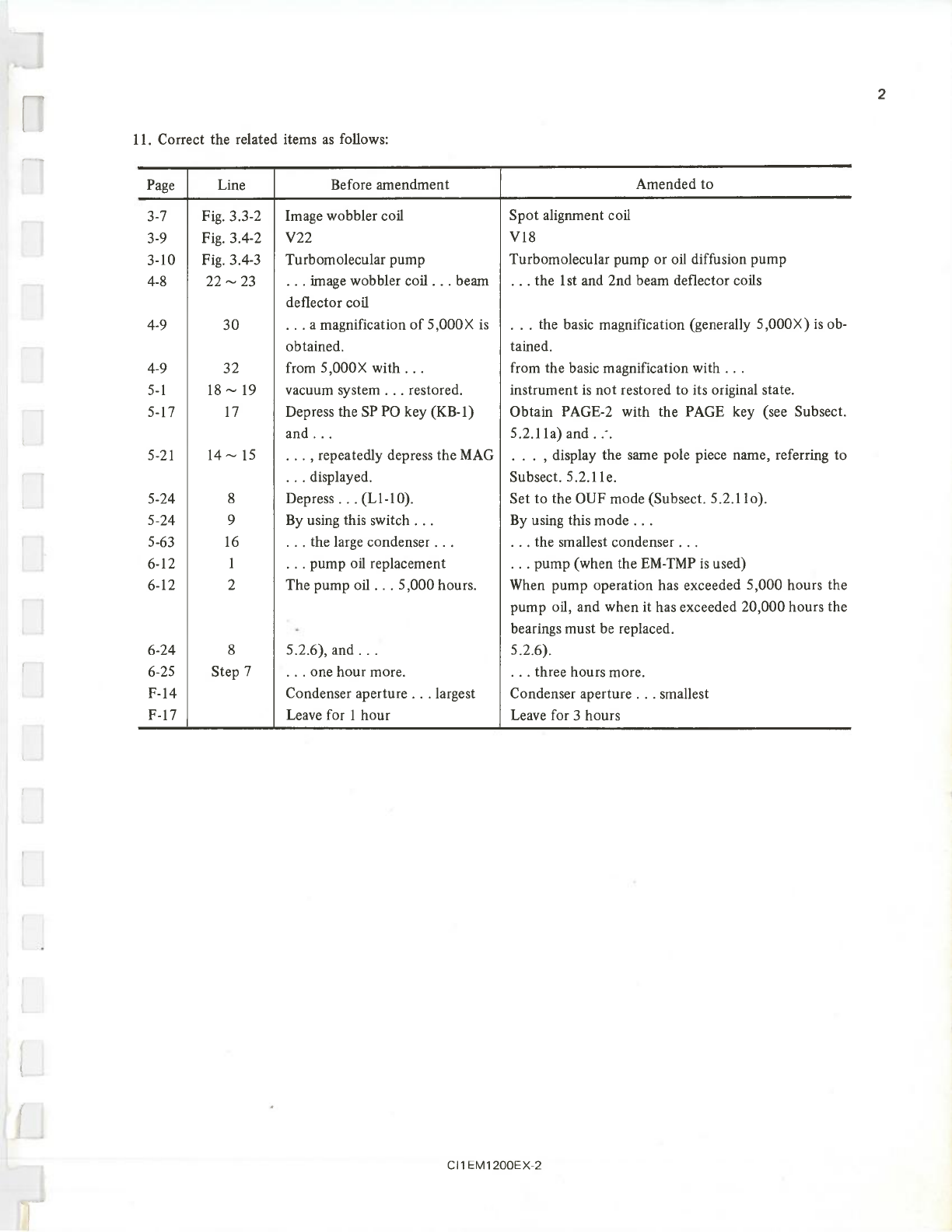
1.
Although the parts listed below are optional accessories, they are described as standard items in this manual
for the sake of convenience. They are: anti-contamination device, refrigerant funnel, refrigerant drainer,
beam stopper, specimen grid case, hexagonal screwdrivers, activated alumina trap and tweezers.
2.
Some controls on control panel LI have been changed as follows:
Ll- C)
:
ROOM LIGHT:
Used for turning on/off the room light.
Ll- C)
:
BRIGHT ZOOM:
For the zoom circuit (see Subsect. 5.2.11q).
Ll- C)
:
BRIGHT 16X:
When this button is switched on, the button lamp lights up and the 2nd con-
denser lens current range, variable by the BRIGHTNESS knob (control panel
L1), enlarges 16 times.
3.
To set the magnification when the MAG2 button (control panel R1) is depressed (this magnification is called
"basic magnification") at 5,000 times, refer to Subsect. 5.2.11r.
4.
The name of the knobs, IW ADJ, on control panel R2 has been changed to "IMAGE WOBBLER ADJ".
5.
The names of the keys, C/R,
1
and
on the keyboard have been changed to "RETURN", "<—" and "—>"
respectively.
6.
Change Steps 8 and 9 in Subsect. 6.1.2 as follows:
Step 8: Depress the GUN AIR button (L2-4). Air is admitted into the anode chamber.
Step 9: Make sure that the PI2 value (indicated on PAGE-1) has increased to 250, then turn the LIFT switch
(L2-1) to ON.
7.
Change Steps 2 and 3 in Subsect. 6.7.7b as follows:
Step 2: Make sure the electron gun has not been lifted and camera chamber door is closed.
Step 3: Switch off the COL AIR button (L2-5).
8.
In Subsect. 6.8, add another step, Step 19, after Step 18.
Step 19: Make the objective lens pole piece name displayed on PAGE-1 coincide with the name of the pole
piece being installed in the objective lens (see Subsect. 5.2.11e).
9.
The aperture disk and aperture holder of the Wehnelt assembly have been removed.
10.
Two types of objective lens aperture, 20-50-80 bunO aperture for the SHP pole piece and 50-100-150 linicb
aperture for the SAP pole piece, have been provided.
8309081