Byeong Cha Page 1 11/24/2009
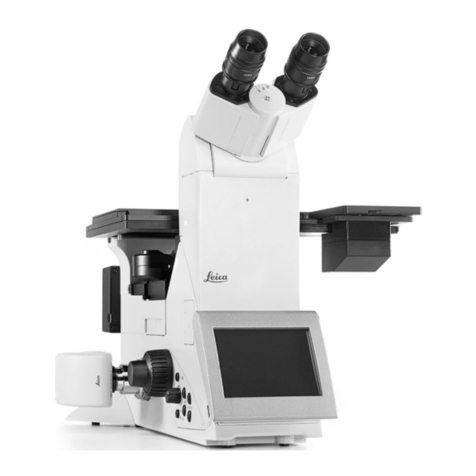
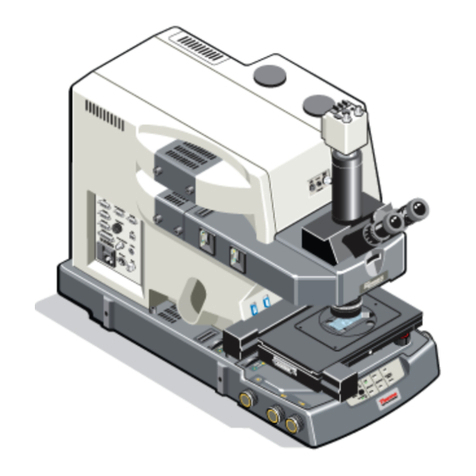
Manual for Leica SP2 Confocal Microscope
Enter you name, the date, the time, and the account number in the user log book.
Things to check before start-up.
•Make sure that your sample slides are clean and sealed. Use Windex and cotton balls or Kimwipes to clean
coverslips. Fixed samples need to be sealed with nail polish.
•Check the objectives. The 10x and 20x objectives are DRY objectives, and should NEVER have oil on them!
There are 40x and 63x OIL immersion objectives, and a 63x WATER immersion objective also on this
microscope. If oil immersion objectives have oil on them, wipe the lens gently only with lens paper. With
the microscope controllerON, you see the information of the objective in position on the reading panel
at the front of the microscope. The order of
the objective lenses in the lens turret is:10X, 20X,
40X Oil, 63X Oil, and 63X Water.
•The 63X objective has aperture collar adjustment
rings that control the size of aperture opening. It
should be wide open for most imaging condition.
If your sample looks dim or the whole view field
is not illuminated evenly when you look through
the eyepieces, turn the collar to open the aperture.
•The field diaphragm and aperture diaphragm in
the excitation path of the mercury lamp are
controlled by round, black dials on the left side
toward the back of the microscope, and control
the fluorescence illumination from the mercury
lamp. For all imaging with the mercury light
source, these should be set wide open by turning all the way counter-clockwise.
Setting up for bright-field and fluorescence
viewing.
Basic Operation of Leica DM IRE2 Microsocpe.
Turn on the microscope controller .
Turn on the mercury arc lamp.
Bright-field viewing:
Place your sample slide with coverslip (preferably #1.5
thickness) facing down to the objective on the stage.
For bright-field viewing, turn on the microscope light by turning the wheel
toward you and turn the VIS-SCAN switch to VIS position (Note:
switch back to Scan position for confocal scanning, see below)
Start with low magnification objectives (10X or 20X) first to find and focus
onto your specimen. The information of the selected objective and their z-