Leica SP8 inverted confocal microscope November 18,2019
•System description:
•DMi8, inverted microscope: BF, Fluo (blue, green, red)
•BF TL Detector (transmitted light image, no additional illumination or time)
•Scanning stage with z-Galvo and Navigator (overviews, selection of regions of
interest,…)
•Scanner with Scanfield Rotation
•2 PMT detectors (photo multipliers)
•1 Hybrid Detector normal (more sensitive and no detector noise nor background)
•1 Hybrid Detector SMD (fast, for FLIM, FALCON)
•Laser lines: 405, White Light Laser (470-670nm, up to 8 laser lines simultaneously)
•FALCON including Pulse Picker for WLL
•LIGHTNING (Deconvolution) for Superresolution and super sensitive imaging
•Objectives:
•Objective PL FLUOTAR 10x/0.30 (overview)
•Objective PL APO 20x/0.75 dry CS2
•Objective PL APO 20x/0.75 IMM Corr CS2 (Oil, water, glycerol, silicon oil)
•Objective PL APO 40x/1.20 Water Corr CS2
•Objective PL APO 63x/1.30 Glycerol Corr CS2
•System Start Up
•Switch on ‘PC-microscope’ button (1). Gives power on the computer and microscope
controller
•Switch on ‘scanner power’ (2)- enables the scanning head
•Switch on ‘Laser power’ (3)
•Turn the key (4) in position ‘on’- enables Laser shutters
•Switch on fluorescent lamp(5) for visual examination
•(if temperature control needed) switch on the temperature controller (6). Set up
temperature for the heating stage (port 2) and objective heater (port 1)
!!!!!! If you are not using Z-galvo stage, always place it UPSIDE DOWN on the
optical table
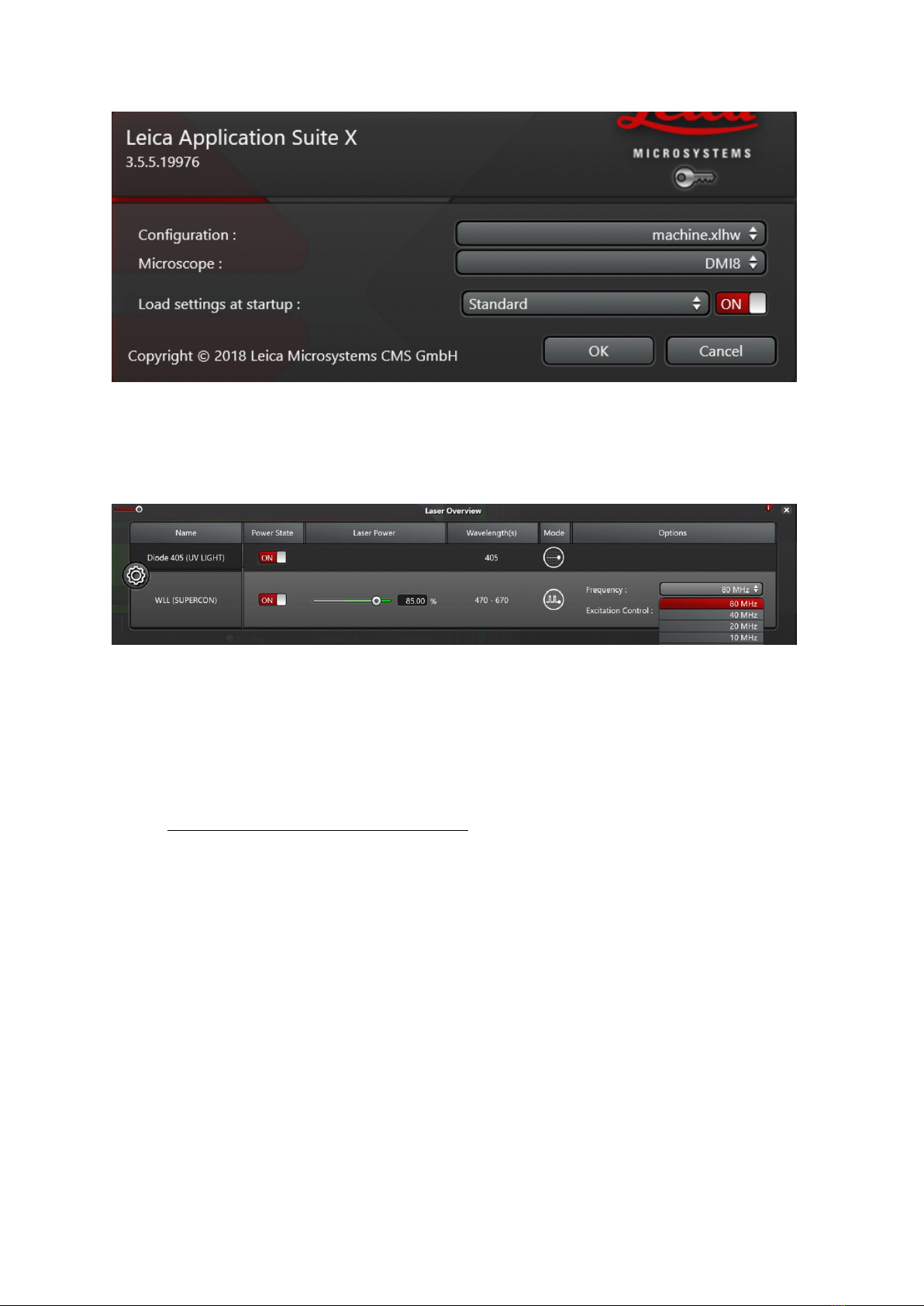
•For imaging: start LAS AF software. Select configuration Machine.xlhw and DMI8
microscope (default). Load of "Standard" settings possible (Fig.1)
•!!!!! Never use "Use last system settings"