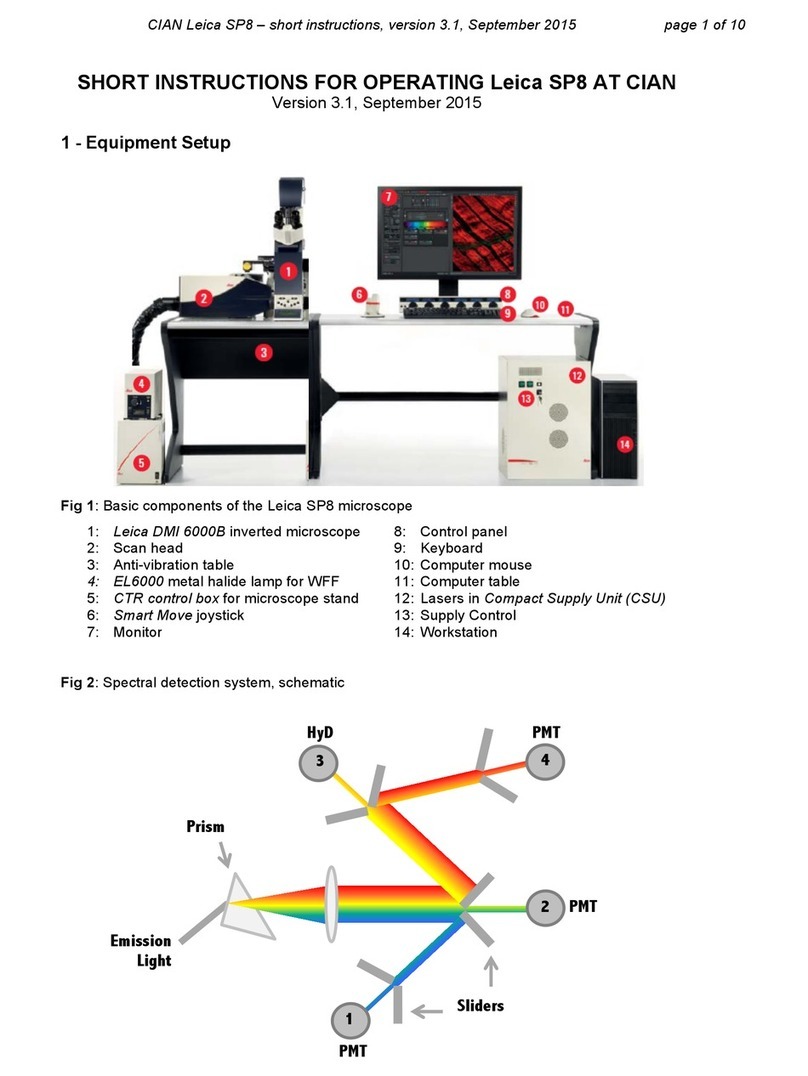
2. select the Acquire Operating step on top left, click the Acquisition tab to set up the data format
A. Acquisition mode panel
defaults to xyz simultaneous scan
seq. button enables sequential scan
Tile scan, and Mark and Find
Best Focus
B. XY panel (click ▶ on upper right corner to expand)
Format: frame resolution defaults to 512x512pixels, max 8192x8192pixels
Speed: line frequency defaults to 400Hz, scan area will be reduced above 700Hz, max 2800Hz
Zoom* and panning
Average and Accumulate
Scan Field Rotation*
Pinhole*: default is 1AU and displays in µm
3. acquire an image (buttons in the lower left pane of LAS AF)
Live: scan, apply accumulate if set
Capture Image: acquire an image at the current z position, apply both average and accumulate if set
Start: acquire all configured scans, e.g., z-stack, stage positions, tile scan, time points, spectral series etc.
*these parameters are also adjustable via the Smart Panel with the default configuration
Optimize acquisition parameters
1. center your prep in the field of view with either BF or FLUO (see Modes of operation in the Appendix)
2. to toggle on scanning, click Live or use the button on the left side of Smart Panel
Caution: if there is no signal in the viewer display Window on the right, DO NOT move the stage
controls (XYZ) or increase the laser power; try the following
i. with BF or FLUO, make sure object of interest is still in focus
ii. open up pinhole to increase the slice thickness
iii. raise PMT gain
iv. lastly, increase laser power
3. bring the object of interest into focus, adjust PMT gain, offset, and laser power to obtain an image
with good dynamic range
Note: the "glow-over, glow-under", Glow (OU) LUT which displays 0 intensity in green and
saturation in blue (255 for 8-bit or 4095 for 12-bit), facilitates this setup
i. top left side of the viewer display Window: activate the Glow (OU) LUT via the Quick
LUT button which cycles through the default , Glow(OU) , and grey LUTs
ii. adjust offset so the pixels in the area you think should be darkest just turn green, usually
around -0.2%; increase the sensitivity of the control panel offset knob may help
iii. vary the PMT gain or laser power so a few pixels in the brightest object of interest just turn
blue
4. with multichannel simultaneous scan, always check for cross-talk between channels by dropping the laser
power of the suspected channel to zero; if cross-talk does present, use sequential scanning