USER
MANUAL
451285-006
Contents
Disclaimers & Warranties.................................................................................................................................................2
1 Overview and Technical Description............................................................................................................... 6
1.1 General description..................................................................................................................................................................................... 6
1.2 Warnings and Cautions.............................................................................................................................................................................7
1.3 Explanation of symbols ............................................................................................................................................................................9
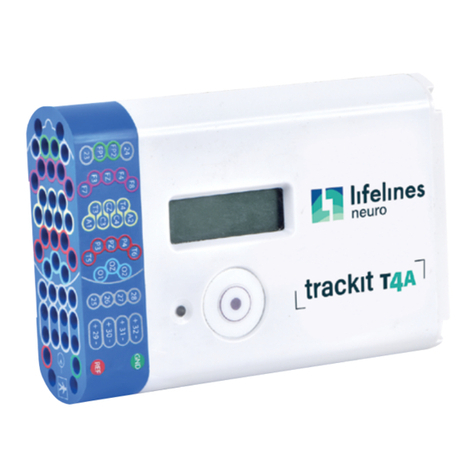
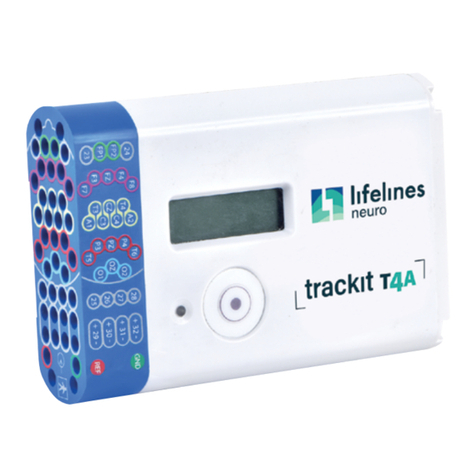
1.4 The Amplifier and its parts ..................................................................................................................................................................10
1.5 Specifications and safety........................................................................................................................................................................11
1.6 Description of the components........................................................................................................................................................12
1.7 Replaceable parts....................................................................................................................................................................................... 14
2 Installation and Maintenance .............................................................................................................................15
2.1 Checks for completeness and integrity .................................................................................................................................... 15
2.2 Environmental parameters for operation................................................................................................................................. 15
2.3 Power supply connections.................................................................................................................................................................. 16
2.4 Battery Operation Time.......................................................................................................................................................................... 16
2.5 Use in the home environment............................................................................................................................................................17
2.6 Use with other equipment ....................................................................................................................................................................17
2.7 Interference..................................................................................................................................................................................................... 18
2.8 Maintenance and cleaning.................................................................................................................................................................. 18
2.9 Disposal of equipment............................................................................................................................................................................ 18
3 Connections and usage .........................................................................................................................................19
3.1 Overview............................................................................................................................................................................................................ 19
3.2 Laptop installation and operation................................................................................................................................................. 20
3.3 Connecting the T4 System................................................................................................................................................................... 21
3.4 Starting the system .................................................................................................................................................................................. 24
3.5 Shutdown of the system.......................................................................................................................................................................25
3.6 Battery replacement and charging.............................................................................................................................................. 26
3.7 Micro-SD Card..............................................................................................................................................................................................27
4 Trackit Software - overview ................................................................................................................................29
Version History ....................................................................................................................................................................47