2piezo smart - manual of use and maintenance
3
piezo smart - manual of use and maintenance
00.0 Introduction
00.1 Foreword
Read this manual carefully before installing the equipment, using it or carrying out
maintenance or any other activities on it.
Always keep this manual within easy reach.
Important: To avoid causing injuries or damage to property, read all the points concerning “Safety
Requirements” contained in this manual particularly carefully. Depending on the level of risk
involved, safety requirements are classed under the following headings:
DANGER (always referred to personal injury)
WARNING (referred to possible damage to property)
The purpose of this manual is to bring the safety requirements, the installation procedures and the
instructions for correct use and maintenance of the device to the knowledge of operators.
The user is not authorised to camper with the equipment under any circumstances.
If any problems are encountered, please contact a Mectron Service Centre.
Anny attempts on the part of the user or of any unauthorised personnel to tamper with or alter the
device will invalidate the warranty and release the manufacturer from any liability in respect of any
harm or damage to persons or property.
The information and illustrations container in this manual are up-dated to the date of publication
indicated on the last page.
MECTRON are committed to continuous up-dating of their products, which may entail changes to
components of the device. If any discrepancies are found between the descriptions container in the
manual and your device, please contact your dealer of the MECTRON Afer-Sale Service.
Using this manual for purposes other to those relating to the installation, use and maintenance of
the equipment is strictly prohibited.
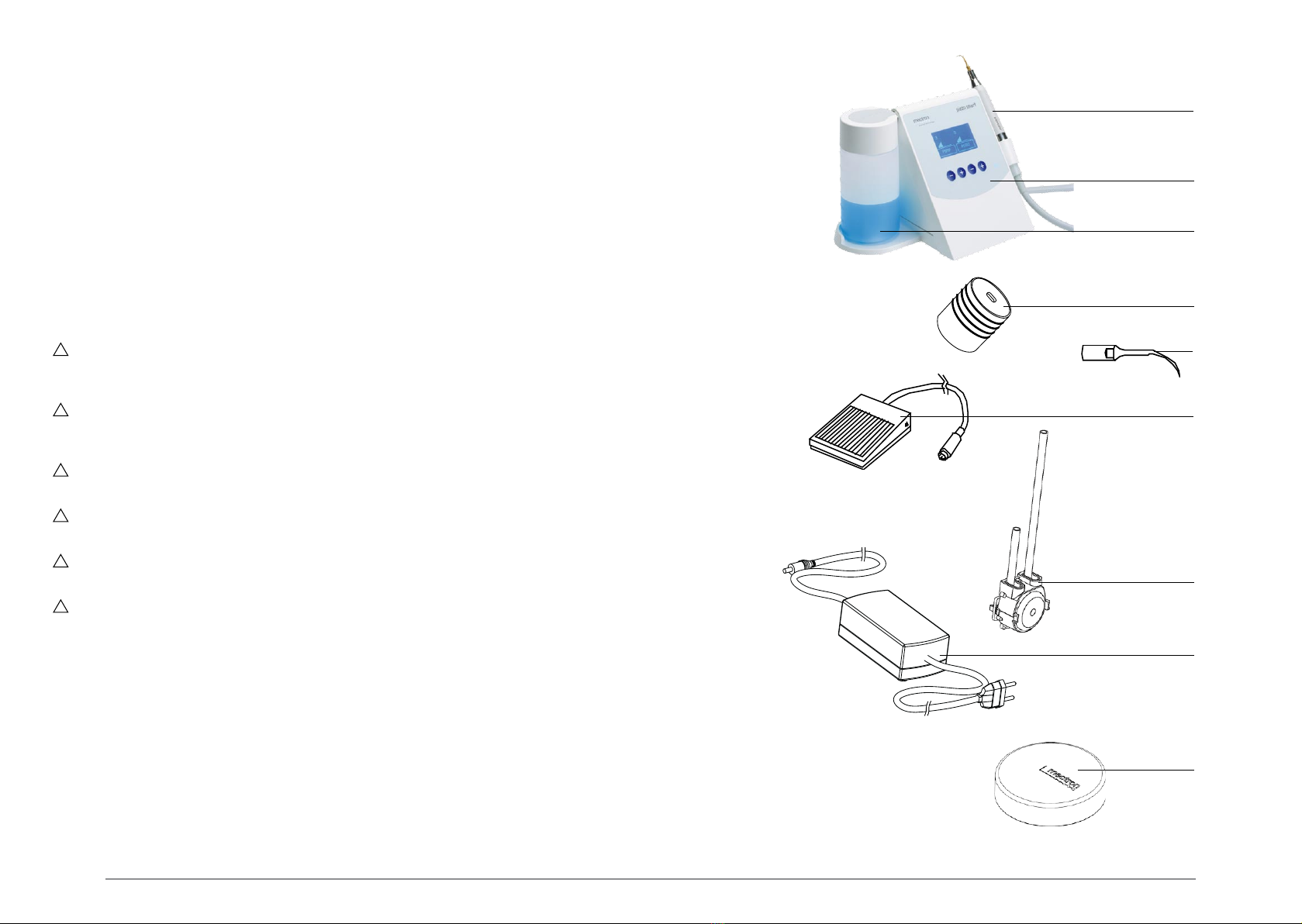
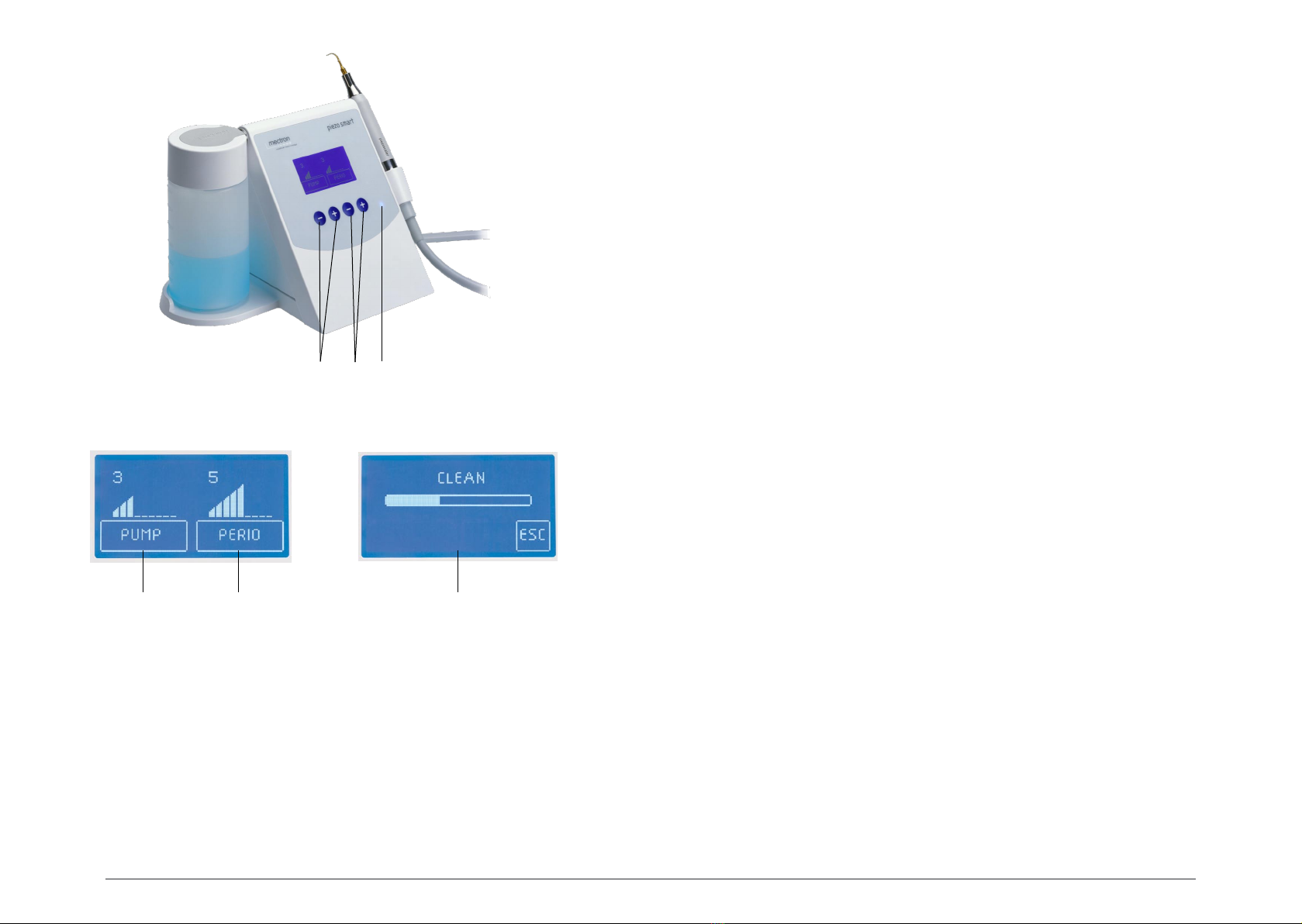
00.2 Description of the device
The piezo smart is an extremely modern ultrasound piezoelectric scaler enabling application the
US technique to dentistry and is manufactured using state-of-the-art technology. The device has an
automatic tuning circuit that offsets wear of the inserts, so that maximum efficiency is ensured at all
times. The handpiece, which can be autoclave sterilised at 135°C, has a titanium resonator and Is
unbreakable.
The piezosmart can use the (optional) Starlight p curing lamp for dental composites. The lamp is
connected to the scaler cord. The device recognises attachment of the lamp automatically.
Summary
00.0 Introduction........................................................................................................................ .....3
00.1 Foreword .................................................................................................................... .....3
00.2 Description of the device ............................................................................................ .....3
00.3 Intended use............................................................................................................... .....4
00.4 Safety Requirements .................................................................................................. .....4
01.0 Identification data.............................................................................................................. .....6
01.1 Identification ............................................................................................................... .....6
01.2 Equipment identification plate..................................................................................... .....6
01.3 Identification place of the scaler handpiece................................................................ .....6
02.0 Testing................................................................................................................................ .....7
02.1 Testing of the equipment ............................................................................................ .....7
03.0 Delivery............................................................................................................................... .....7
03.1 Delivery of the device ................................................................................................. .....7
03.2 List of material included in the standard supply.......................................................... .....8
04.0 Installation.......................................................................................................................... .....8
04.1 Safety requirements during installation ...................................................................... .....8
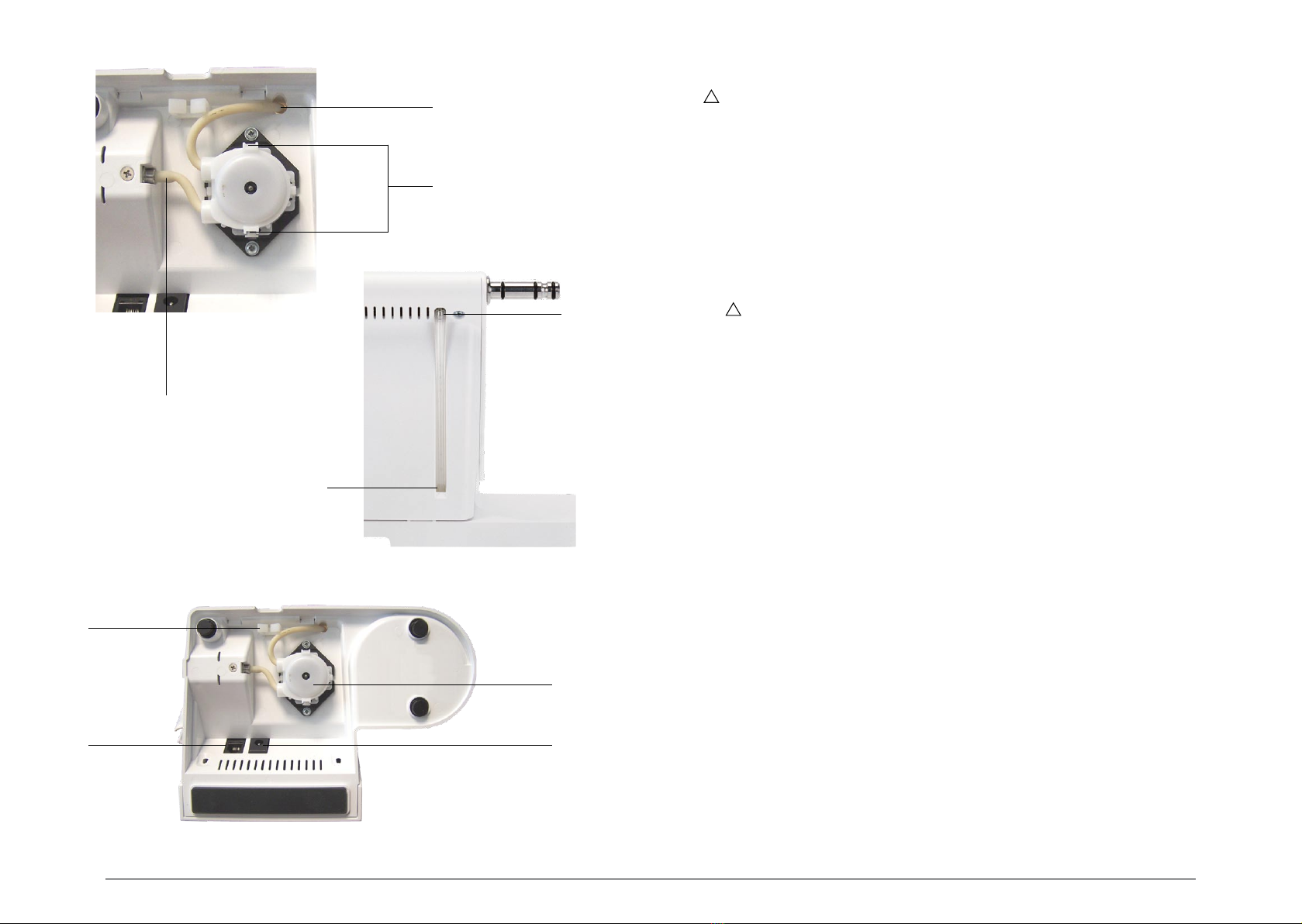
04.2 Installation of the peristaltic pump .............................................................................. ...11
04.3 Connection of the device ............................................................................................ ...11
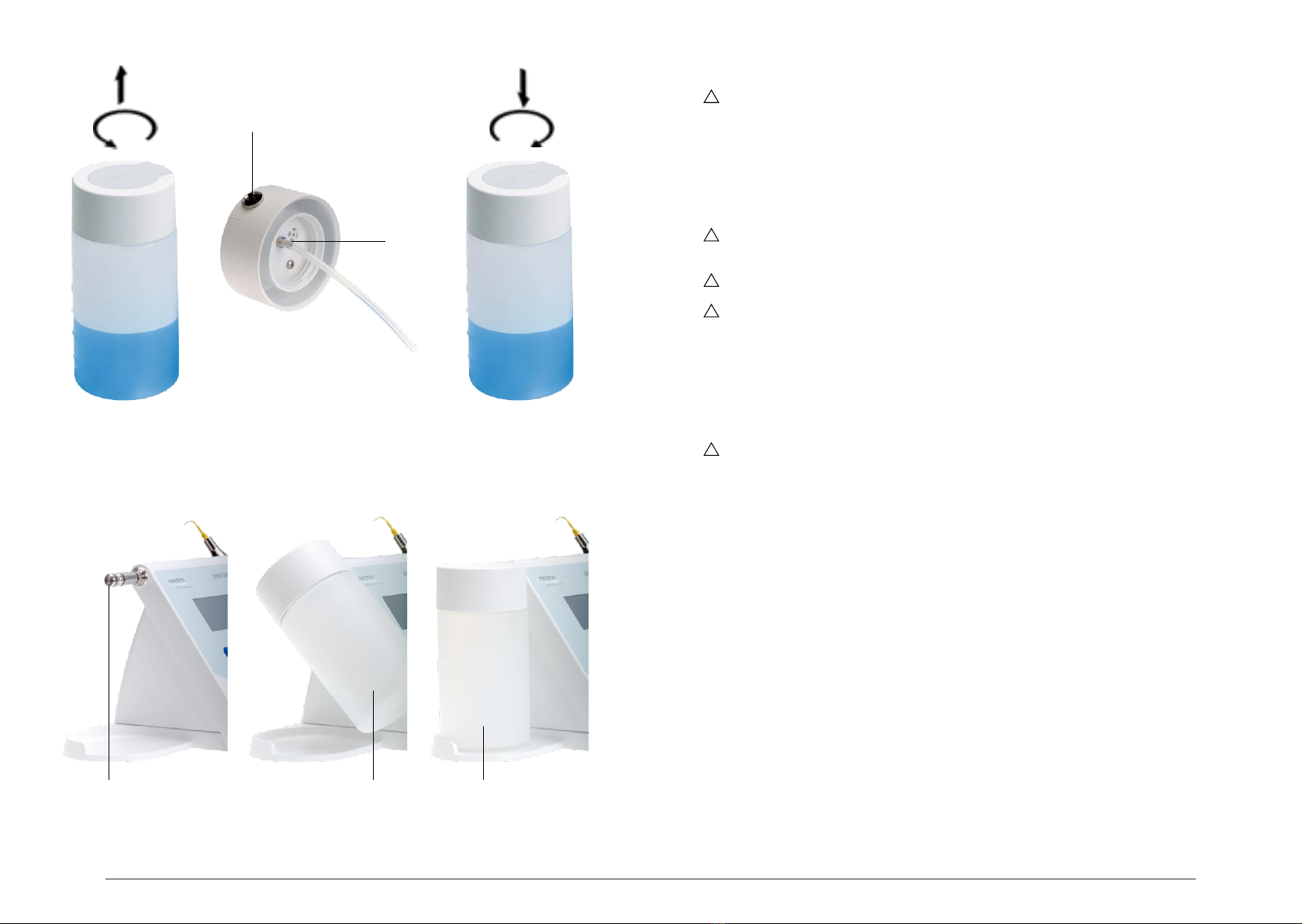
04.4 Preparing the bottle .................................................................................................... ...13
04.5 Installing the bottle on the equipment......................................................................... ...13
04.6 Removing the bottle from the device .......................................................................... ...13
05.0 Use...................................................................................................................................... ...14
05.1 Controls ...................................................................................................................... ...14
05.2 Switching on and off ................................................................................................... ...15
05.3 Description of the display and functions ..................................................................... ...15
05.4 Safety Requirements during use ................................................................................ ...16
05.5 Safety and alarm circuits ............................................................................................ ...17
05.6 Instructions for use ..................................................................................................... ...18
05.7 Checking the inserts for wear ..................................................................................... ...18
06.0 Cleaning, disinfection and sterilisation........................................................................... ...19
06.1 CLEAN Function - Cleaning of the liquid circuit.......................................................... ...19
06.2 Cleaning and disinfecting the housing of the device .................................................. ...19
06.3 Sterilisation procedure ............................................................................................... ...20
06.4 Autoclave sterilisation of the handpiece ..................................................................... ...20
06.5 Autoclave sterilisation of the inserts ........................................................................... ...21
06.6 Autoclave sterilisation of the torque wrench for tightening the inserts........................ ...21
07.0 Scheduled maintenance ................................................................................................... ...21
07.1 Cleaning of the water circuit ....................................................................................... ...21
07.2 External power supply unit ......................................................................................... ...21
07.3 Replacement of the peristaltic pump .......................................................................... ...21
08.0 Procedures for disposal and precautions ...................................................................... ...22
09.0 Inserts for Mectron scaler and how to use them............................................................ ...22
10.0 Trouble-shooting............................................................................................................... ...23
11.0 Symbols.............................................................................................................................. ...25
12.0 Technical specifications ................................................................................................... ...26
12.1 Electromagnetic compatibility EN 60601-1-2............................................................... ...27
13.0 Warranty............................................................................................................................. ...31