2 / 32 Piezosurgery // GB
MANUAL OF USE AND MAINTENANCE
Summary
00.0 - INTRODUCTION ............................................................................................................................... 3
00.1 - Foreword ...................................................................................................................................... 3
00.2 - Description of the Device ............................................................................................................. 3
00.3 - Intended Use................................................................................................................................ 4
00.4 - Safety requirements ..................................................................................................................... 4
01.0 - IDENTIFITION DATA ........................................................................................................................ 6
01.1 - Identification data......................................................................................................................... 6
01.2 - Data Plate of the Device .............................................................................................................. 6
01.3 - Data Plate of the Scaler Handpiece.............................................................................................. 6
02.0 - TESTING .......................................................................................................................................... 7
02.1 - Testing of the equipment .............................................................................................................. 7
03.0 - DELIVERY ........................................................................................................................................ 7
03.1 - Delivery of the apparatus ............................................................................................................. 7
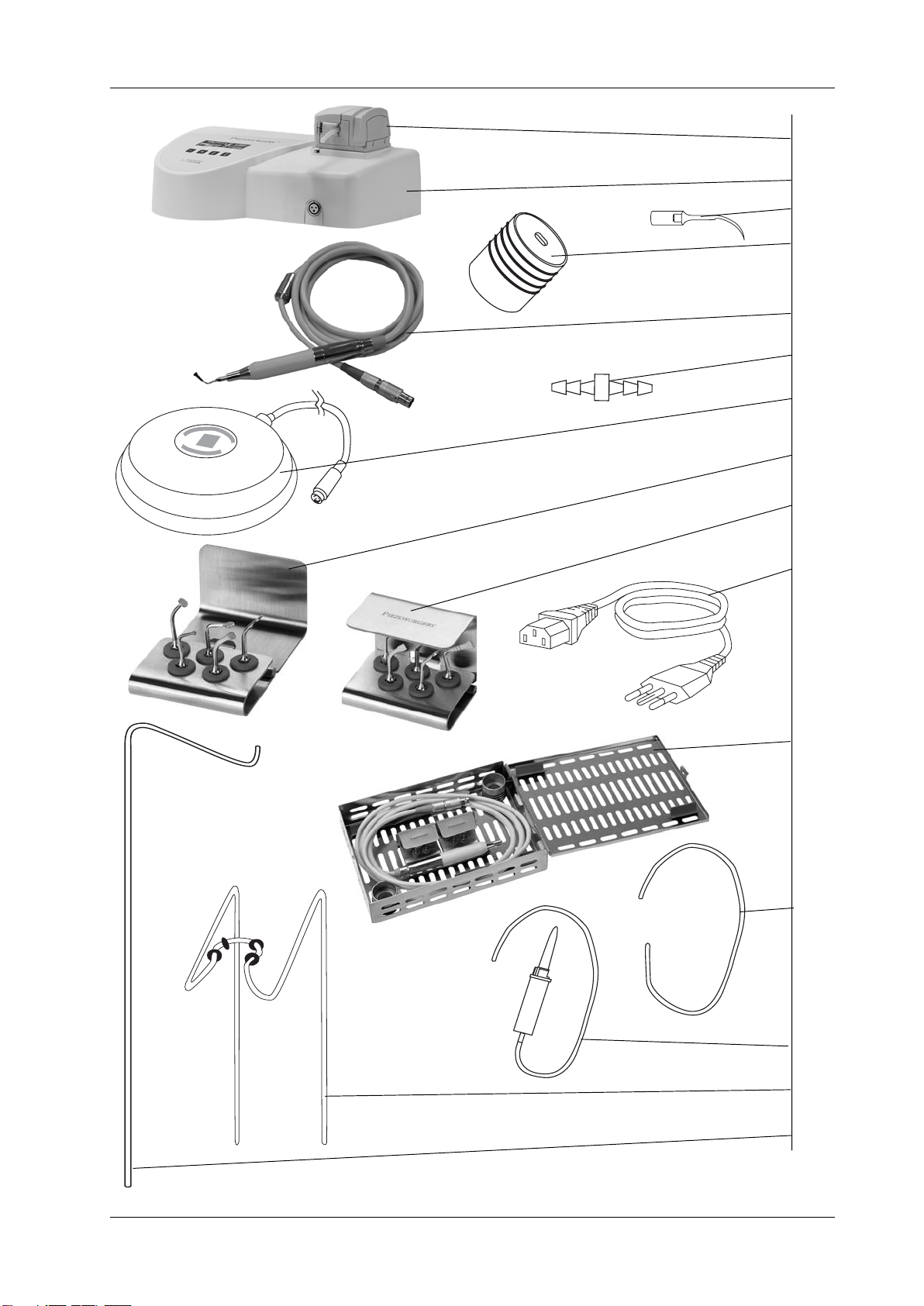
03.2 - List of Materials included in the Standard Supply ......................................................................... 8
04.0 - INSTALLATION ................................................................................................................................ 8
04.1 - Safety Requirements during Installation ....................................................................................... 8
04.2 - Initial Installation ........................................................................................................................ 10
04.3 - Connecting the Accessories....................................................................................................... 10
05.0 - USE ................................................................................................................................................ 13
05.1 - Controls ..................................................................................................................................... 13
05.2 - Switching the Device ON and OFF ............................................................................................. 13
05.3 - Description of the Display and Functions ................................................................................... 14
05.4 - Safety Requirements during Use ................................................................................................ 16
05.5 - Protection Systems and Alarms ................................................................................................. 17
05.6 - Instructions for Use .................................................................................................................... 18
05.7 - Rules for Keeping the Device in Proper Working Order ............................................................... 18
06.0 - CLEANING, DISINFECTION AND STERILISATION ....................................................................... 19
06.1 - CLEAN function – Cleaning of the liquid circuit ..........................................................................19
06.2 - Cleaning and disinfecting the casing of the apparatus ................................................................ 19
06.3 - Sterilisation procedure ................................................................................................................ 20
06.4 - Autoclave sterilisation of the handpiece ..................................................................................... 20
06.5 - Autoclave sterilisation of the inserts .......................................................................................... 21
06.6 - Autoclave sterilisation of the wrench for tightening the inserts ................................................... 21
06.7 - Autoclave sterilisation of the peristaltic pump tube .................................................................... 22
06.8 - Autoclave sterilisation of the connection between cord and peristaltic pump tube connection .... 22
07.0 - REGULAR MAINTENANCE ............................................................................................................ 23
07.1 - Shelf Storage ............................................................................................................................. 23
07.2 - Power-supply Cable ................................................................................................................... 23
08.0 - REPLACEMENT OF THE FUSES .................................................................................................. 23
09.0 - PROCEDURES AND PRECAUTIONS FOR DISPOSAL ................................................................. 24
10.0 - THE INSERTS ................................................................................................................................ 24
11.0 - SYMBOLS ...................................................................................................................................... 25
12.0 - TROUBLESHOOTING .................................................................................................................... 26
13.0 - TECHNICAL DATA ......................................................................................................................... 29
14.0 - GUARANTEE.................................................................................................................................. 31