EN
3
01 ÛINTRODUCTION
01 ÛINTRODUCTION
Carefully read this manual before proceeding
with the cleaning and sterilization operations,
and always keep it within reach.
IMPORTANT: to prevent harm to persons
or damages to objects, read all the “Safety
precautions”present in the manual with
special care. Depending on their degree
of seriousness, the safety precautions are
classified with the following indications:
WARNING
(always refers to personal injury)
CAUTION
(refers to possible damage to property)
The purpose of this manual is to make
the operator knowledgeable of the safety
precautions, the installation procedures,
and the instructions for a correct use and
maintenance of the device and its accessories.
Use of this manual for purposes other than
those strictly tied to cleaning and sterilization
of the device is forbidden.
The information and illustrations in this
manual are updated as of the date of issue
reported on the last page.
Mectron is committed to continuously
update its products with possible
modifications to device components.
In case you uncover discrepancies between
what described in this manual and the device
in your possession, contact your Retailer or the
After-Sales Service of Mectron for clarification
and support.
Limitations on the number of cleaning and
sterilization cycles: Cleaning and sterilization
cycles repeated over time have minimum
effects on the instruments examined in this
Manual.The life expectancy of the products is
usually determined by their consumption and
by damages caused from their use.
WARNING: The operators who perform
the cleaning and sterilization operations
must be adequately protected and trained
WARNING: Infections control.
First use: All new and repaired accessories
are supplied in NON STERILE conditions.
Before use, and after each treatment, they
must be cleaned and sterilised in strict
compliance with the instructions given in
the Cleaning and Sterilization Manual.
Subsequent uses: After every treatment,
clean and sterilize all the reusable parts
and accessories, following the instructions
provided in the Cleaning and Sterilization
Manual.
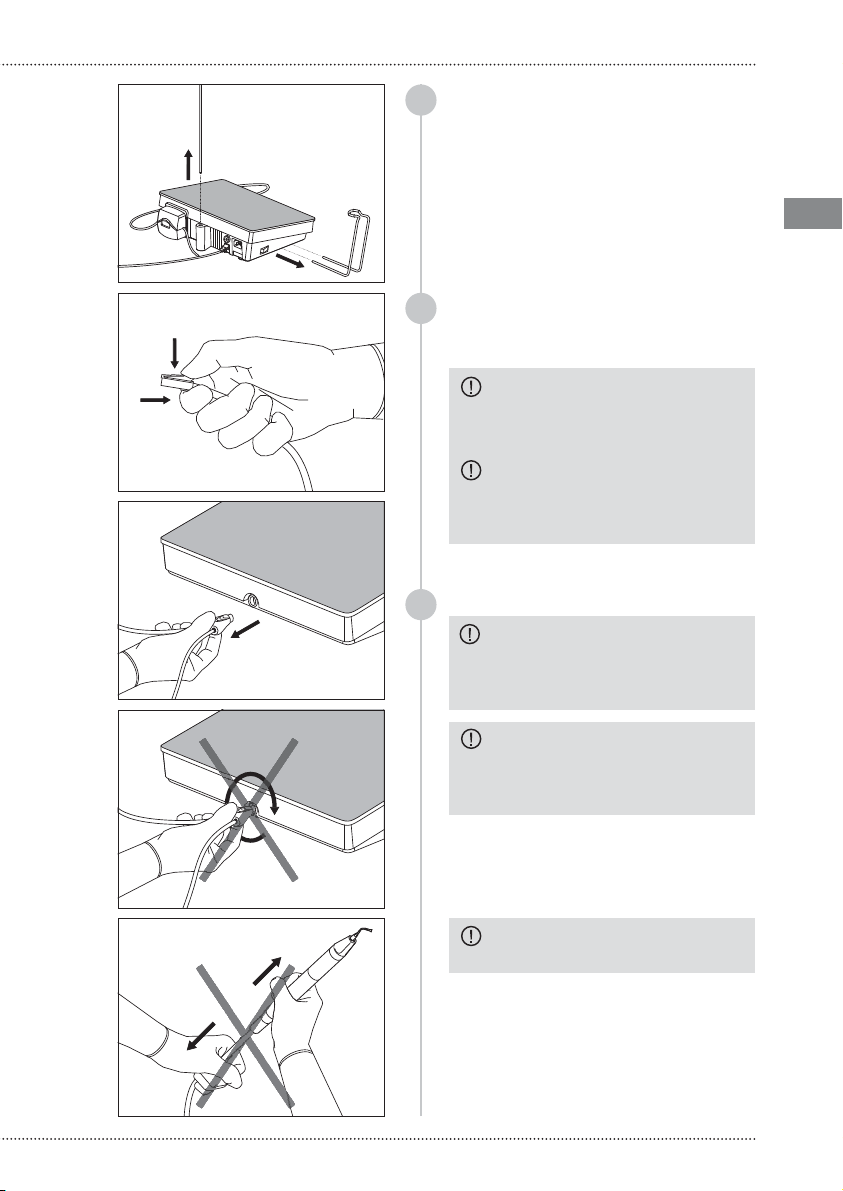
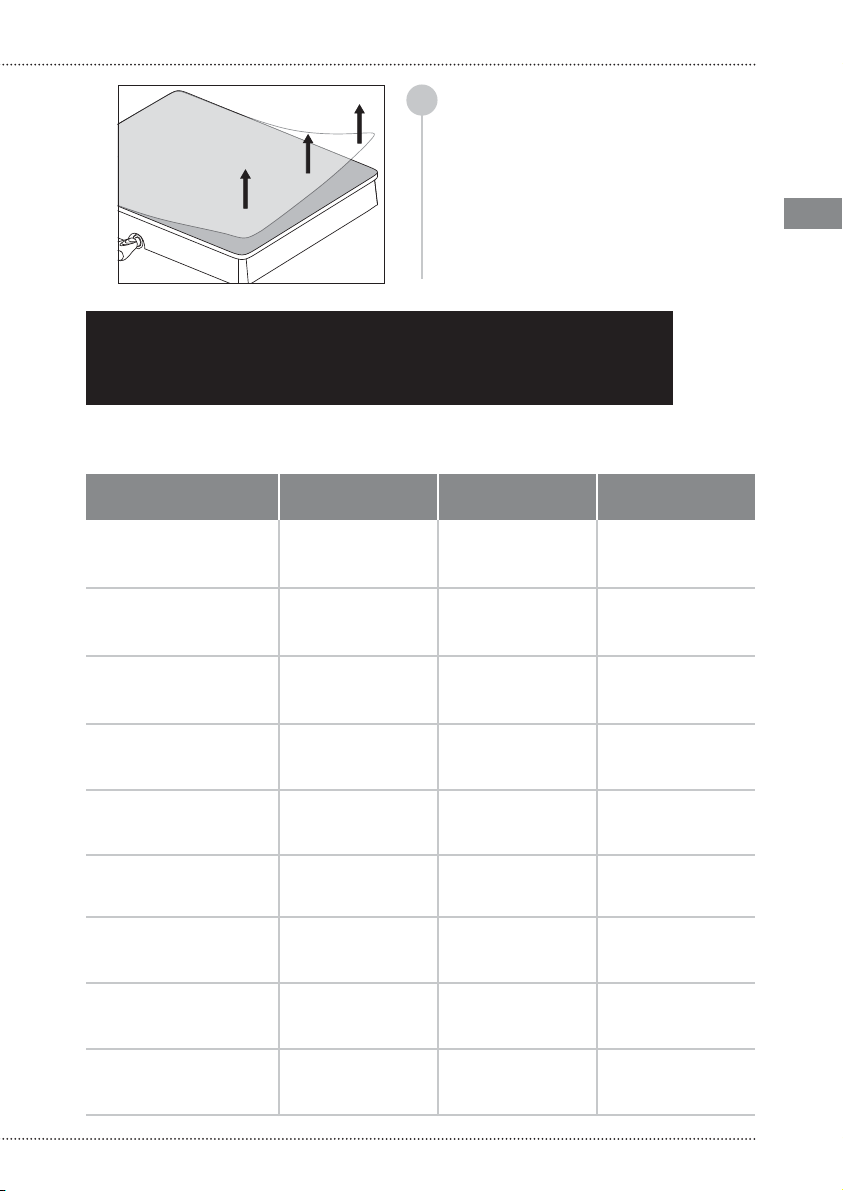
WARNING: The cleaning processes
must be commenced immediately after
each use. Do not allow that contaminated
instruments dry up before starting
the cleaning and sterilization process.
To eliminate organic remains such as
blood, bone, and other, use an enzymatic
detergent with neutral pH (pH7),
immediately after use.
CAUTION: Do not use metallic brushes
or abrasive sponges during the cleaning
processes,because they damage the
surfaces of the treated parts and the
finishes of the inserts. Only use brushes
with soft nylon bristles.
CAUTION: The detergent solution must
be completely removed from the treated
parts to prevent accumulation of chemical
residues.
CAUTION: During cleaning procedures
only use detergents with pH 6-9.
CAUTION: If you intend to disinfect,
we recommend that you use water-based
disinfectant solutions with a neutral pH
(pH 7). Alcohol-based disinfectant solutions
and hydrogen peroxide are contra-
indicated, because they can fade the color
and/or damage the plastic materials.This
also holds true for chemical products such
as acetone and alcohol. Always rinse with
sterile water to preserve the disinfection.
WARNING: Do not use tap water, unless
where explicitly indicated.
WARNING: Reusable accessories that
must be forwarded to an Authorized
Mectron Service Center. All the parts must
be cleaned and sterilized in accordance to
the procedures described in this Manual,
before they are sent to an Authorized
Mectron Service Center.
Incorrectly treated and bio-contaminated
parts will not be accepted by the
Authorized Mectron Service Centers.