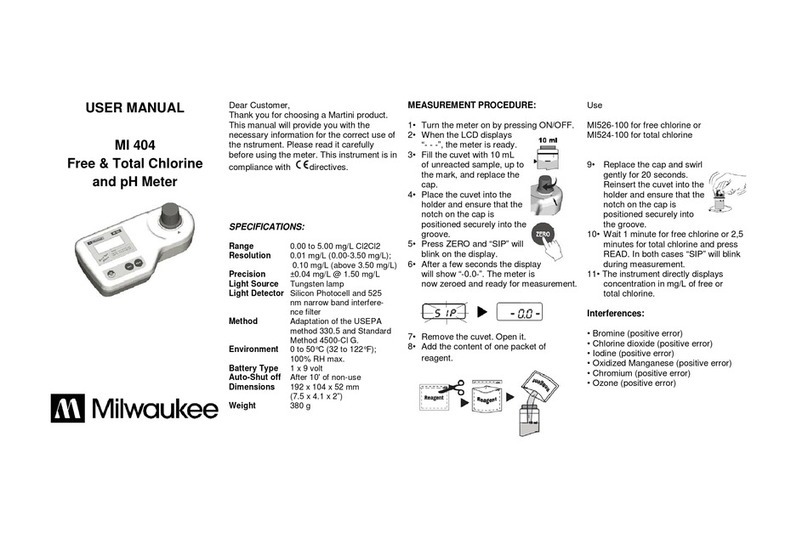
6MI455 Minititrator for Wine Analysis
2. GENERAL DESCRIPTION
The MI455 is a low-cost, easy to use, microprocessor-based automatic titrator. It has a
simple and reliable peristaltic pump that ensure high dosing repeatability. By performing
pump calibration with the provided Milwaukee standards, the instrument accuracy is
assured.
The instrument comes with a pre-programmed analysis method designed for Free and
Total Sulphur Dioxide measurements on wine samples. The instrument has a powerful
and eective built-in algorithm to analyze the shape of the electrode response and to
determine the reaction completion. This algorithm automatizes the analysis, makes all
the necessary calculations and assures a simple and eective interface for the user.
By simply pressing the START STOP button, the instrument will automatically make the
titration up to the equivalence point. The result is immediately displayed in convenient
units, then the instrument is ready for another titration.
Significance of Use
An important reason for adding SO₂ is to avoid oxidation. When there is oxygen around,
SO₂ itself becomes oxidized before phenol compounds in the wine, and so acts as an
oxygen scavenger. Also SO₂ suppresses the activity of enzymes that cause browning and
other problems.
What is really protecting your wine is molecular SO₂. When you add SO₂, depending
of circumstances, some of it immediately becomes bound. The relationship between
the amount of added SO₂ and the amount of SO₂ remaining free is complex. It is clear,
however, that it is largely governed by the total SO₂ content of the wine. The rate of binding
decreases as the free SO₂ concentration increases. The exact relationship between free
and bound (total – free) SO₂ will vary from wine to wine.
Below 30-60 ppm, 33% to 50% of SO₂ addition becomes bounded. What remains is
called “free” and it is divided in two parts. The larger, and relatively ineective free part is
“bisulphite” (HSO3¯). The smaller part of the free is the active molecular SO₂. The amount
of molecular SO₂ in your wine depends both on the level of free SO₂ present as well as
pH. For instance, at pH 3.2, the amount of free SO₂ for 0.8 ppm molecular SO₂ is 22 ppm.
AtpH3.5, you will need 43 ppm free – essentially double.
Free SO₂ concentration (ppm) for 0.8 ppm molecular SO₂:
pH 3.0 3.1 3.2 3.3 3.4 3.5 3.6 3.7 3.8 3.9
Free SO₂ 14 18 22 28 35 44 55 69 87 109