INDEX
1. INTRODUCTION..................................................................................................................................................................................................................3
1.1. INTRODUCTORY REMARKS .............................................................................................................................................................................................3
1.1.1. General Aspects..................................................................................................................................................................................................................3
1.1.2. Safety Aspects.....................................................................................................................................................................................................................3
1.1.3. Legal Aspects .....................................................................................................................................................................................................................3
1.1.4. Environmental Aspects...................................................................................................................................................................................................... 3
1.2. DATA REGISTRATION........................................................................................................................................................................................................3
1.2.1. Equipment and customer registration / Service file ........................................................................................................................................................4
1.2.2. Configuration register........................................................................................................................................................................................................4
1.2.3. Product documentation .....................................................................................................................................................................................................4
1.2.4. Technical modifications ....................................................................................................................................................................................................4
1.2.5. Product evaluation .............................................................................................................................................................................................................4
1.2.6. Additional Information ......................................................................................................................................................................................................4
1.3. GENERAL...............................................................................................................................................................................................................................5
1.3.1. Technical Data...................................................................................................................................................................................................................5
1.3.1.1. MiniSpir unit ......................................................................................................................................................................................................................5
1.4. STANDARDS APPLIED ........................................................................................................................................................................................................5
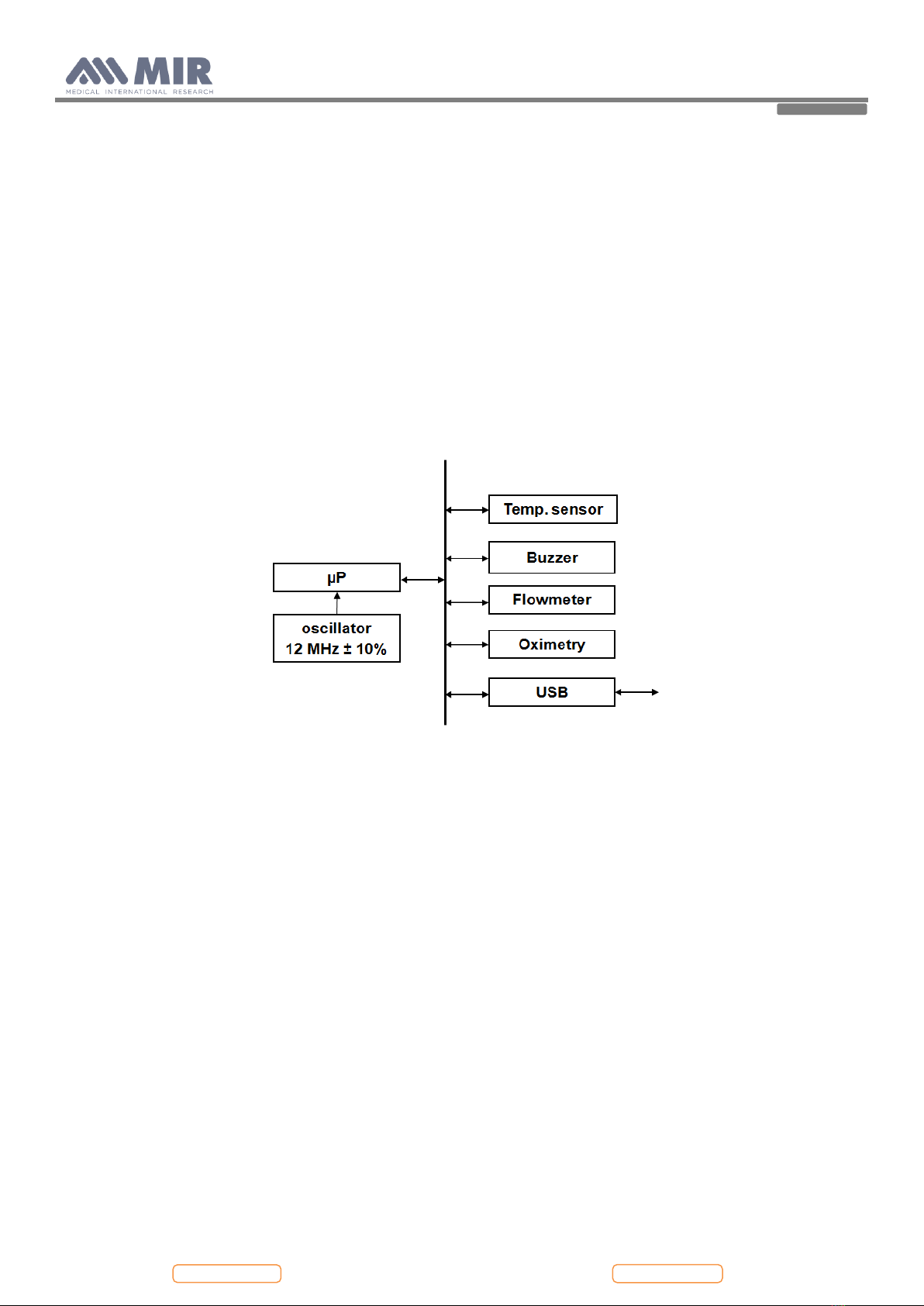
2. HARDWARE DESCRIPTION ..............................................................................................................................................................................................6
2.1. MAIN BOARD MODULE .....................................................................................................................................................................................................6
2.1.1. Room temperature sensor..................................................................................................................................................................................................6
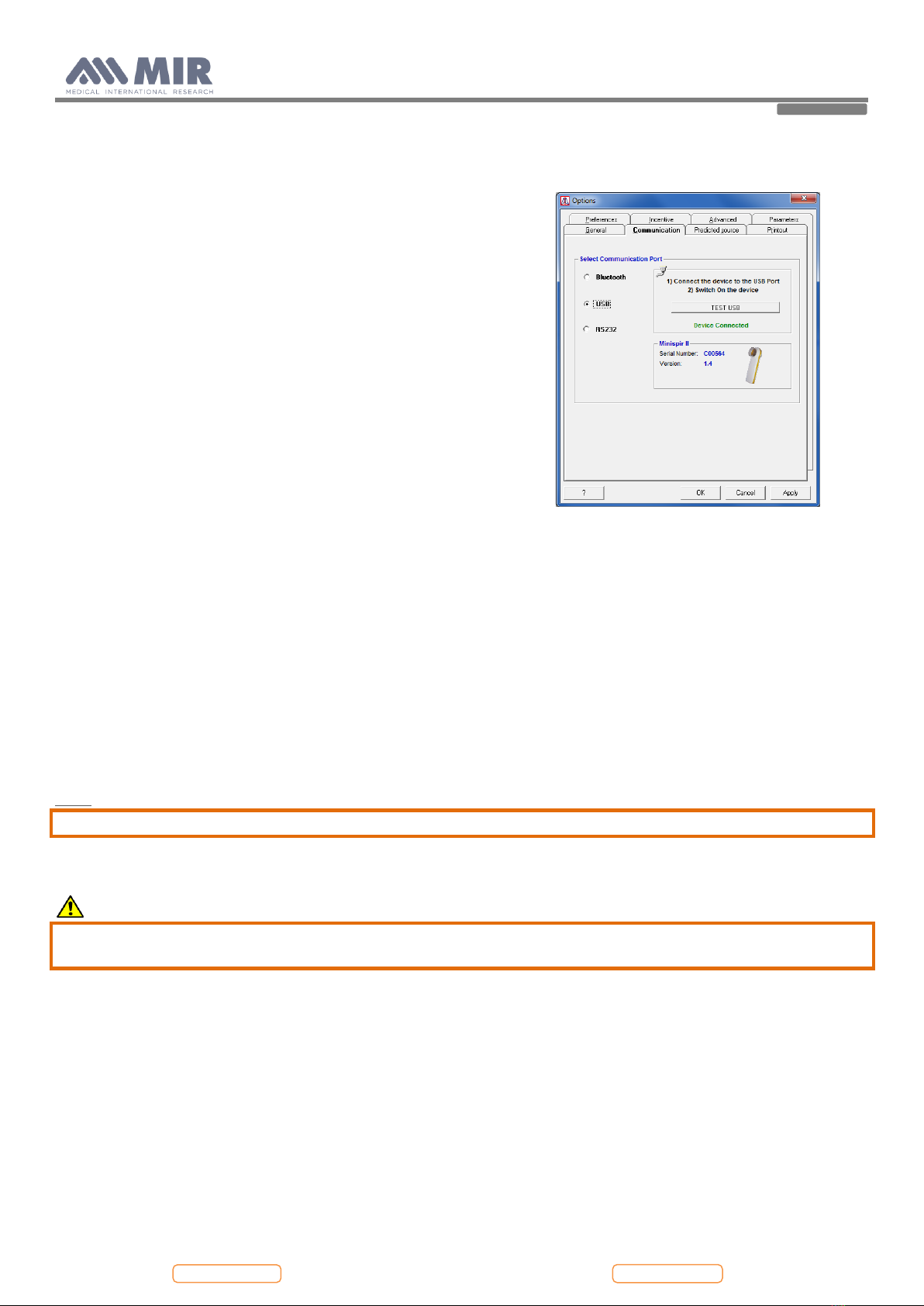
2.1.2. USB communication port..................................................................................................................................................................................................7
2.1.3. Oximetry port .....................................................................................................................................................................................................................7
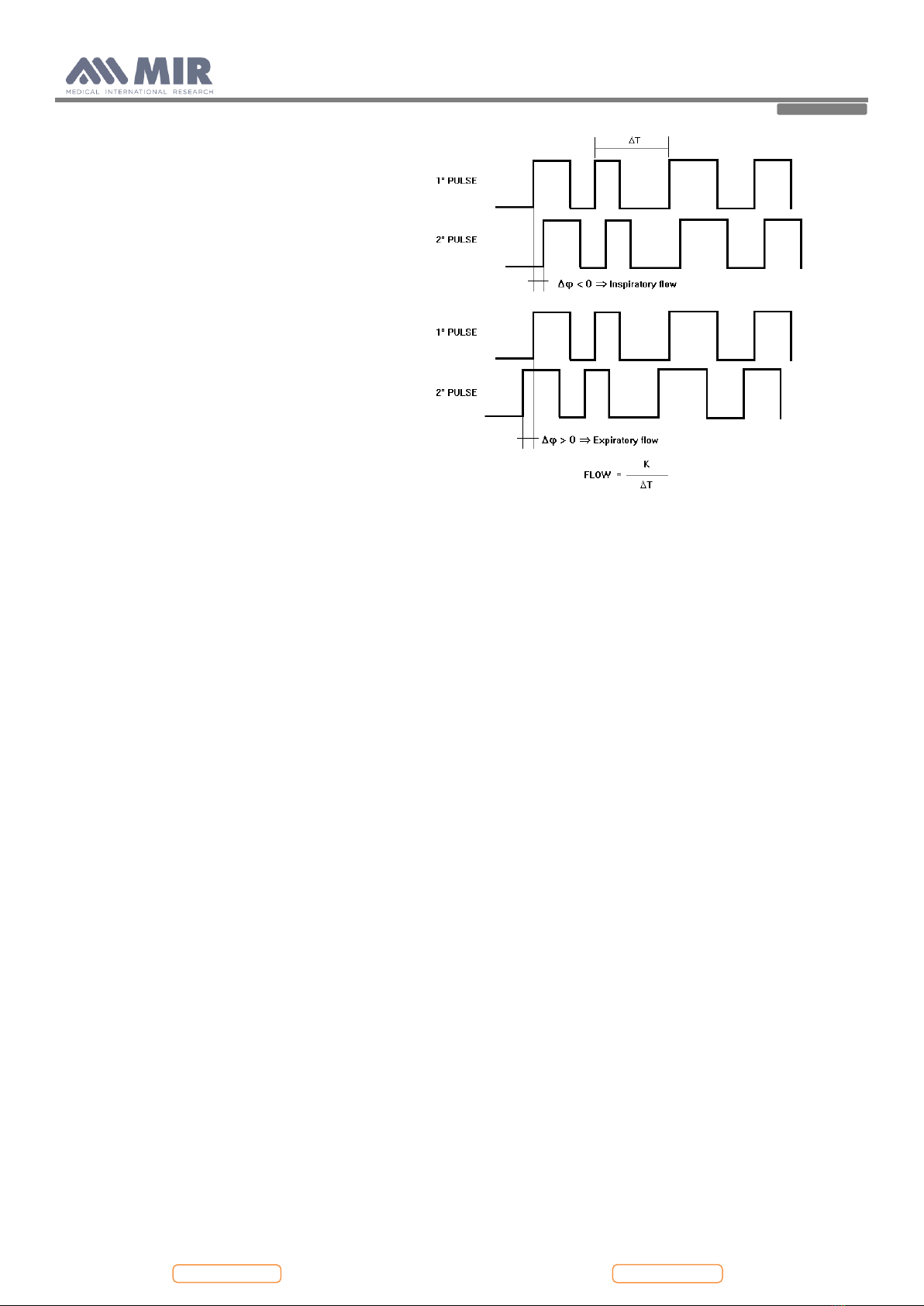
2.2. TURBINE FLOWMETER.....................................................................................................................................................................................................7
3. MAINTENANCE ...................................................................................................................................................................................................................8
3.1. GENERAL...............................................................................................................................................................................................................................8
3.2. TEST EQUIPMENT ..............................................................................................................................................................................................................8
3.3. CHECKLIST............................................................................................................................................................................................................................8
3.3.1. Functional test....................................................................................................................................................................................................................9
3.3.1.1. Software version .................................................................................................................................................................................................................9
4. REPLACEMENT PROCEDURES .......................................................................................................................................................................................9
4.1. General .....................................................................................................................................................................................................................................9
4.2. Cover....................................................................................................................................................................................................................................... 10
4.2.1. Opening the device .......................................................................................................................................................................................................... 10
4.3. PCBs and components .......................................................................................................................................................................................................... 10
4.3.1. Replacing the USB cable in case of MiniSpir Light....................................................................................................................................................... 10
4.3.2. Turbine..............................................................................................................................................................................................................................11
4.3.2.1. Cleaning the reusable turbine...........................................................................................................................................................................................11
4.3.2.2. Calibration of the reusable turbine.................................................................................................................................................................................. 12
4.3.3. Internal software upgrade procedure .............................................................................................................................................................................. 12
4.4. Oximeter module ................................................................................................................................................................................................................... 13
4.4.1. Replacing of the oximetry module .................................................................................................................................................................................. 14
4.5. Testing procedures ................................................................................................................................................................................................................ 14
4.5.1. Testing procedures .......................................................................................................................................................................................................... 14
5. SPARE PARTS....................................................................................................................................................................................................................... 17
5.1. ORDERING .......................................................................................................................................................................................................................... 17
5.2. DELIVERY............................................................................................................................................................................................................................ 17
5.2.1. Ordering PCB's................................................................................................................................................................................................................ 17
5.2.2. Warranty claims................................................................................................................................................................................................................ 17
5.3. RETURN SHIPMENTS ...................................................................................................................................................................................................... 18
6. TROUBLESHOOTING....................................................................................................................................................................................................... 18
6.1. The device does not switch on .............................................................................................................................................................................................. 18
6.2. The device does not measure spirometry at all .................................................................................................................................................................... 18
6.3. The device does not measure spirometry correctly.............................................................................................................................................................. 18
6.4. The device does not measure oximetry at all ....................................................................................................................................................................... 19
6.5. The device does not measure oximetry correctly ................................................................................................................................................................. 19
6.6. The data communication via USB does not function .......................................................................................................................................................... 19
6.7. Index of components ............................................................................................................................................................................................................. 19
APPENDIX A: SPARE PARTS LIST .............................................................................................................................................................................................. 20
APPENDIX B: SERVICE INFO'S (Product Change Notes) ........................................................................................................................................................ 24
APPENDIX C.................................................................................................................................................................................................................................... 25
ANNEX.............................................................................................................................................................................................................................................. 27