Page 3 of 136
Table of contents
1General safety instructions.......................................................................... 9
1.1 Explanation of the safety symbols used .......................................................... 9
1.1.1 Symbols used in instruction manual.................................................... 9
1.1.2 Symbols appearing on the device ....................................................... 9
1.1.3 Symbols appearing on the LiquoGuard®7 display ............................. 10
1.1.4 Symbols indicated on the retail packaging ........................................ 12
1.2 Explanation of the conventions applied......................................................... 14
1.3 Manufacturer’s responsibility......................................................................... 14
1.4 Equipotential bonding.................................................................................... 14
1.5 Owner´s duty of care..................................................................................... 15
1.6 Additional accessories .................................................................................. 16
1.7 Single use..................................................................................................... 16
1.8 Declaration on DEHP.................................................................................... 17
1.9 Application during defibrillation and for electrosurgical devices..................... 17
2Purpose ....................................................................................................... 18
2.1 Indications of temporary intrathecal CSF drainage........................................ 18
2.2 Indications for permanent CSF drainage....................................................... 19
2.3 Contraindications.......................................................................................... 19
2.4 Complications ............................................................................................... 19
2.5 Combination with other products, catheters and cannulas ............................ 20
2.6 Patient population & residual risk.................................................................. 21
2.7 Essential performance .................................................................................. 21
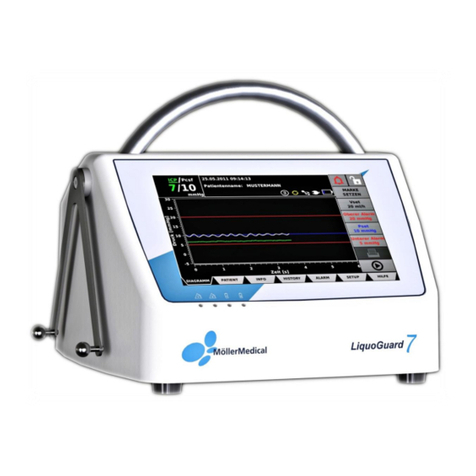
3Product description.................................................................................... 22
3.1 Touchscreen................................................................................................. 23
3.2 Bag holder .................................................................................................... 23
3.3 Universal bracket.......................................................................................... 23
3.4 Pump............................................................................................................ 23
3.5 LEDs............................................................................................................. 23
3.6 Connection options....................................................................................... 24
3.7 User interface ............................................................................................... 25
3.7.1 Tab bar............................................................................................. 26
3.7.2 Softkeys............................................................................................ 27
3.7.3 Keyboard screen............................................................................... 27
3.8 Diagram dialog field ...................................................................................... 29
3.8.1 Diagram............................................................................................ 30
3.8.2 Info bar ............................................................................................. 32