4Precautions for Use
•Cardiogreen and other intravascular dyes, depending on the concentration,
may affect the accuracy of the SpO2measurement.
•Ear Clip and Reflectance sensors are not recommended for pediatric or
neonatal use. The accuracy of these sensors has not been established for
pediatric or neonatal use.
•Do not immerse the 2500A or NONIN sensors in liquid, and do not expose
the device or components to excessive moisture or liquids.
•Do not use caustic or abrasive cleaning agents on the 2500A or the sensors.
•The oximeter sensor might not work on cold extremities due to reduced
circulation. Warm or rub the finger to increase circulation, or reposition the
sensor.
•The 2500A isaprecisionelectronic instrument and mustberepaired bytrained
NONIN personnel only.
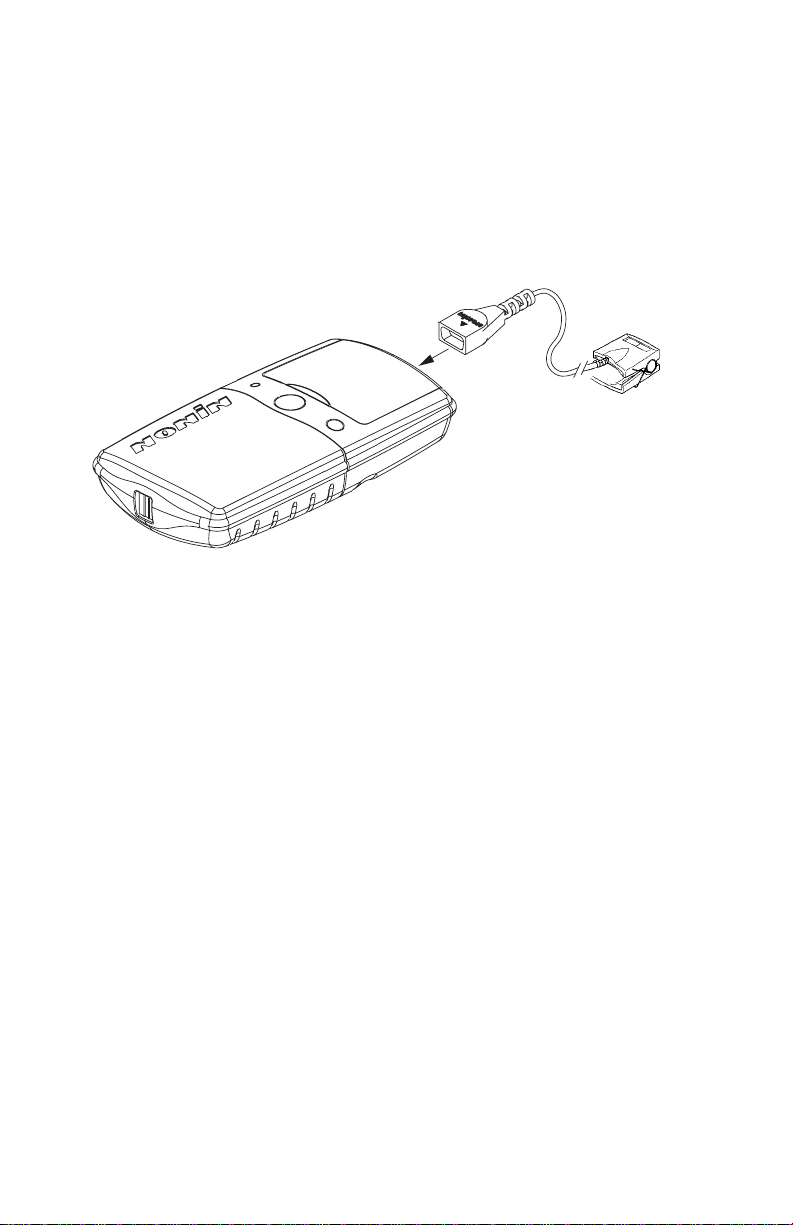
•Replace the batteries as soon as possible after a low battery indication. Always
replace the batteries with fully-charged batteries.
•Use only NONIN-specified battery types with this device.
•Do not mix fully charged and partially charged batteries at the same time. This
may cause the batteries to leak.
•Do not remove any covers other than the battery cover when replacing
batteries. There are no user-serviceable parts inside other than the replaceable
batteries.
•Follow local governing ordinances and recycling instructions regarding
disposal or recycling of the device and device components, including batteries.
•Batteries may leak or explode if used or disposed of improperly.
•Remove the batteries if the 2500A will be stored for more than 1 month.
•This equipment complies with International Standard EN 60601-1-2:2001 for
electromagnetic compatibility for medical electrical equipment and/or
systems. This standard is designed to provide reasonable protection against
harmful interference in a typical medical installation. However, because of the
proliferation of radio-frequency transmitting equipment and other sources of
electrical noise in healthcare and other environments, it is possible that high
levels of such interference due to close proximity or strength of a source might
disrupt the performance of this device. Medical electrical equipment needs
special precautions regarding EMC, and all equipment must be installed and
put into service according to the EMC information specified in this manual.
•In compliance with theEuropean Directive on Waste Electrical and Electronic
Equipment (WEEE) 2002/96/EC, do not dispose of this product as unsorted
municipal waste. This device contains WEEE materials; please contact your
distributor regarding take-back or recycling of the device. If you are unsure
how to reach your distributor, please call Nonin for your distributor’s contact
information.
•Portable and mobile RF communications equipment can affect medical
electrical equipment.
Cautions (Continued)